In the global fight against the COVID-19 pandemic, mRNA vaccines have emerged as groundbreaking heroes. These remarkable shots have not only revolutionized the way we combat viruses but have also ignited hope for a post-pandemic world. Join us as we explore the science, safety, and significance of COVID-19 mRNA vaccines, unravelling the innovative technology that has changed the course of history.
mRNA vaccines represent a breakthrough in immunization and have become an essential tool in the fight against infectious diseases. This innovative vaccine technology has made headlines recently for its pivotal role in developing rapidly effective vaccines against COVID-19.
In this comprehensive guide, we will explore what sets mRNA vaccines apart from conventional vaccine types, how they work to train our immune systems, and the ways this platform can revolutionize vaccine development and global public health in the future.
What is mRNA Vaccine?
mRNA vaccines are a new type of vaccine that teaches our cells how to make a protein that will trigger an immune response inside our bodies. Here are some critical points about mRNA vaccines:
- mRNA stands for messenger RNA. mRNA is a molecule that provides instructions for our cells to make proteins.
- In mRNA vaccines, scientists synthesize a piece of mRNA with instructions for making a harmless piece of the “spike protein” found on the surface of the COVID-19 virus.
- When this mRNA is injected as a vaccine, our cells follow the instructions to make the spike protein. It causes our immune system to produce antibodies and activate other immune cells to fight off what it thinks is an infection.
- The mRNA breaks down quickly after it delivers the instructions. It never enters the cell’s nucleus, where our DNA is kept. Our cells break down and get rid of the mRNA soon after using the instructions.
- The immune response trained by the mRNA vaccine protects us if we are later exposed to the actual COVID-19 virus. When the real virus tries to infect us, our immune system is already prepared to respond.
- mRNA vaccines provoke a strong immune response without exposing us to any form of the live virus. It makes them safe and effective against COVID-19.
So, mRNA vaccines give our cells instructions for how to make a harmless viral protein that trains our immune system to fight the real virus. The mRNA itself breaks down quickly after delivering its message.
mRNA vaccines work by introducing a synthetic form of messenger RNA (mRNA) into the body that contains instructions for cells to produce specific proteins found in a virus. The mRNA messenger molecules essentially provide a genetic “blueprint” that teaches our cells how to generate viral proteins that can trigger an immune response and build immunity against future infection.
Key Components of mRNA Vaccines
- Messenger RNA (mRNA): A modified mRNA sequence encoding instructions for cells to produce target viral proteins.
- Lipid Nanoparticles: Lipid (fatty) nanoparticles that encase and protect the fragile mRNA strands for delivery into cells
- Viral Spike Proteins: harmless spike proteins generated by cells based on mRNA instructions that provoke an immune reaction.
This mechanism differs significantly from traditional vaccines that inject weakened or inactive forms of viruses to trigger immunity. mRNA vaccines can stimulate an immune response and build protective antibodies without using any form of the live virus itself.
Advantages of mRNA vaccines:
- It does not contain any live virus; there is no risk of infection from the vaccine
- Allow rapid vaccine design and scalable manufacturing
- High efficacy demonstrated in clinical trials
- Early data shows strong real-world effectiveness
mRNA technology provides an innovative platform for developing vaccines faster and more efficiently than conventional methods. Instead of requiring months or years to produce weakened viruses or proteins in cells or eggs, mRNA vaccines can be designed and synthesized within weeks based on the genetic sequence of the target virus. This speed and flexibility gave mRNA vaccines a leg up in the race to develop a vaccine against the novel coronavirus.
How do mRNA vaccines work?
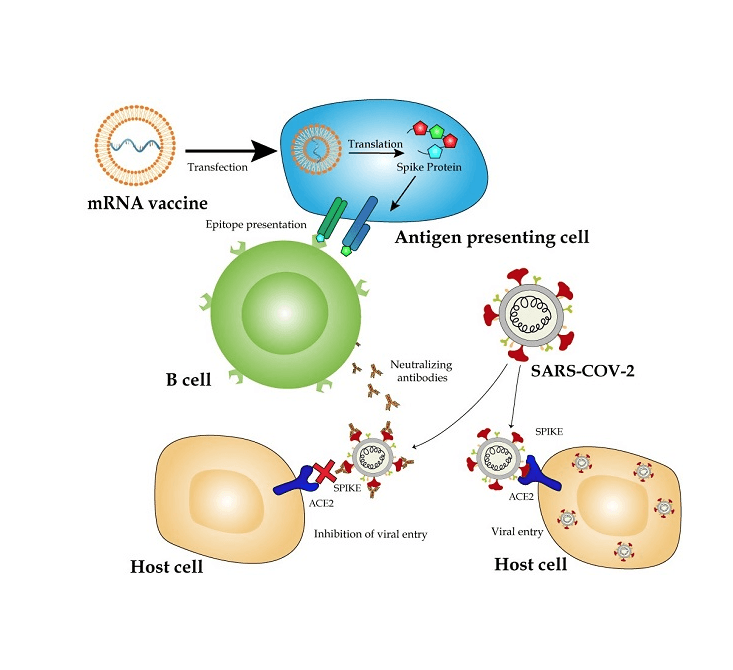
Here is a step-by-step explanation of how mRNA vaccines work:
- Scientists choose a target protein that is unique to the virus they want to vaccinate against. For COVID-19, the spike protein on the virus surface was selected.
- They determine the genetic code (mRNA sequence) needed to produce that protein. The mRNA is like an instruction manual for cells to build the protein.
- The mRNA is encapsulated in a lipid nanoparticle coating. This coating protects the mRNA and helps deliver it to human cells after vaccination.
- When the vaccine is injected, the lipid nanoparticles fuse with the outer membrane of muscle cells near the injection site. It allows the mRNA to enter the cells.
- Inside the cell, the mRNA instructions are ‘read’ by ribosomes, which are cellular machines that build proteins.
- The ribosomes construct many copies of just the target viral protein. These proteins are released from the cell and prompt an immune response.
- Immune cells detect the foreign viral protein and activate B-cells to produce antibodies against it. Other immune cells are also activated.
- The mRNA is quickly broken down within days after it is used. However, the immune cells remain activated and remember how to fight the virus.
- If the real virus tries to infect the body later, the immune system is already primed and quickly destroys it with antibodies and immune cells. It prevents illness.
So, in summary, the mRNA enables human cells to construct parts of the viral protein, which trains the immune system to identify and destroy the real virus. The mRNA itself is short-lived and never enters the cell nucleus.
Milestones in the Development of mRNA Vaccines
The technology behind mRNA vaccines has been developing for decades since early concepts emerged in the 1970s. However, the successful development of the COVID-19 mRNA vaccines from Pfizer-BioNTech and Moderna represents humans’ first approved use of this technology.
Some key milestones in mRNA vaccine research include:
- 1978: Researchers propose that RNA could induce protein production within cells.
- 1990s: mRNA vaccine technology begins development, pioneered by researchers like Ingmar Hoerr and Steve Pascolo.
- 2005: It’s been demonstrated that lipid nanoparticles can deliver mRNA effectively into cells.
- 2009: Moderna Therapeutics was founded to advance mRNA therapeutics and vaccines.
- 2013-2016: Nanoparticle delivery systems and manufacturing advances enable rapid mRNA vaccine development.
- 2020: Pfizer-BioNTech and Moderna COVID-19 vaccines receive emergency use authorization from the FDA.
- 2021-Present: Over 1.5 billion mRNA vaccine doses have been administered globally, providing real-world solid evidence of safety and efficacy.
While the concept had been around for years, critical innovations in mRNA delivery and manufacturing unlocked the technology’s potential for vaccines starting in the 2010s. The COVID-19 pandemic accelerated research, funding, and demand for solutions, enabling the first mRNA products to cross the finish line.
Like all vaccines in the United States, mRNA vaccines require authorization or approval from the Food and Drug Administration (FDA) before they can be used.
Now proven effective against COVID-19, mRNA vaccines could pave the way for rapid responses to future pandemics and developing vaccines against other infectious diseases.
How mRNA vaccines played against COVID-19 Pandemic?
The urgency of the COVID-19 crisis required vaccine development at an unprecedented pace. mRNA vaccines emerged as frontrunners and enabled the first authorized COVID-19 vaccines within less than a year—a process that generally takes ten years or more.
Pfizer-BioNTech and Moderna harnessed mRNA technology to produce safe and highly effective COVID-19 vaccines in record time.
Individuals not previously vaccinated with any COVID-19 vaccine may get two doses administered three weeks apart.
Highlights include
- Pfizer-BioNTech BNT162b2: Approved for emergency use in December 2020, it was the first COVID-19 vaccine authorized in the Western world. Clinical trials showed 95% efficacy with mild side effects. Over 1 billion doses have been administered globally.
- Moderna mRNA-1273: Authorized in December 2020, shortly after Pfizer’s vaccine. Trials demonstrated 94% efficacy and a robust immune response. Over 500 million doses have been administered.
- In fact, scientists at both Pfizer-BioNTech and Moderna drew on their experience developing mRNA cancer vaccines to create their coronavirus vaccines.
- Authorization of Pfizer-BioNTech COVID-19 Vaccine for emergency use in individuals 6 months through 11 years of age to include the 2023-2024 formula.
These mRNA covid-19 vaccines set new records from development to authorization in under a year. Their success has demonstrated the strengths of mRNA technology and re-shaped expectations for vaccine development timelines in the future.
Vaccines types
Vaccines are essential tools in preventing and controlling infectious diseases. There are various types of vaccines, each designed to stimulate the immune system and protect against specific pathogens. In the context of COVID-19, several different types of vaccines have been developed to combat the virus. Here’s an overview of both the general types of vaccines and the different types of COVID-19 vaccines:
Types of Vaccines
- Inactivated or Killed Vaccines: These vaccines contain pathogens (viruses or bacteria) that have been rendered non-functional, typically through heat or chemical treatments. Examples include the inactivated polio vaccine (IPV) and hepatitis A vaccine.
- Live Attenuated Vaccines: Live vaccines use weakened forms of pathogens that can still replicate but cause no or only mild disease. Examples include the measles, mumps, and rubella (MMR) vaccine and the yellow fever vaccine.
- Subunit, Recombinant, or Protein Vaccines: Subunit vaccines use only a part of the pathogen, such as a protein or surface antigen, to stimulate an immune response. Examples include the hepatitis B vaccine and the human papillomavirus (HPV) vaccine.
- Viral Vector Vaccines: These vaccines use a harmless virus (not the one causing the disease) to deliver genetic material from the target pathogen, stimulating an immune response. The Oxford-AstraZeneca COVID-19 vaccine and the Johnson & Johnson COVID-19 vaccine are examples of viral vector vaccines.
- Nucleic Acid Vaccines: Nucleic acid vaccines, such as mRNA (messenger RNA) and DNA vaccines, introduce genetic material that encodes specific antigens from the pathogen. They instruct the body to produce these antigens, eliciting an immune response.
Different Types of COVID-19 Vaccines
- mRNA Vaccines: These vaccines, including the Pfizer-BioNTech and Moderna COVID-19 vaccines, use a small piece of the virus’s mRNA to instruct cells to produce the spike protein found on the SARS-CoV-2 virus. The immune system then generates a response against this protein.
- Viral Vector Vaccines: The Oxford-AstraZeneca, Johnson & Johnson’s Janssen, and Sputnik V COVID-19 vaccines use harmless viruses (not SARS-CoV-2) to deliver genetic material that encodes the spike protein, prompting an immune response.
- Inactivated Vaccines: Some COVID-19 vaccines, like Sinopharm and Sinovac’s CoronaVac, use inactivated virus particles to stimulate an immune response. These vaccines contain whole SARS-CoV-2 virus particles that have been rendered non-infectious.
- Protein Subunit Vaccines: Novavax’s COVID-19 vaccine, for example, utilizes a protein subunit approach. It contains a piece of the spike protein that triggers an immune response.
- Other Approaches: Several experimental COVID-19 vaccines use different strategies, including protein subunit vaccines with adjuvants (enhancers of the immune response), DNA-based vaccines, and other novel technologies. These are still in various stages of development and approval.
Each of these COVID-19 vaccine types has its unique advantages, production requirements, and potential side effects. The choice of vaccine depends on factors such as availability, local regulations, and individual health conditions. It’s important to consult with healthcare professionals to determine the most suitable vaccine for you.
How do mRNA vaccines work in the body? A Step-by-Step Process
Now that we have covered the basics of what mRNA vaccines are, let’s dive deeper into how they provoke an immune response within the body:
Step 1: Vaccine Administration
- The mRNA vaccine is injected, usually into the upper arm muscle.
- The vaccine contains the mRNA genetic material encapsulated in lipid nanoparticles for protection and efficient delivery into cells.
Step 2: Cellular Uptake
- The lipid nanoparticles interact with cell membranes and are taken into muscle cells near the injection site.
- It releases the mRNA into the cells.
Step 3: Translation
- Inside the cell cytoplasm, the mRNA instructions are “read” by ribosomes that begin synthesizing target viral spike proteins.
- These proteins match those found on the surface of the coronavirus.
Step 4: Immune Activation
- The immune system recognizes the spike proteins as foreign, activating B and T cells in response.
- B cells begin producing neutralizing antibodies that can bind to the viral spikes on the actual coronavirus.
- T cells learn to identify and destroy infected cells by displaying viral protein fragments.
Step 5: Adaptive Immunity
- This adaptive immune response protects against future infection with the coronavirus by removing infected cells and neutralizing viruses before they can replicate.
- Memory B and T cells can rapidly respond if the body reencounters the virus.
By mimicking a viral infection, mRNA vaccines can teach the immune system how to rapidly mount defenses against the actual live virus in the future. It spares the vaccine recipient from getting sick while generating robust and durable protection.
Benefits and Strengths of mRNA Vaccines
The unique mechanism of mRNA vaccines provides inherent advantages over traditional vaccine platforms regarding speed, flexibility, efficacy, and safety.
a. Speed of Development
- mRNA vaccines can be designed and manufactured rapidly based on the genetic sequencing of a target virus.
- This capability enabled the development of the first COVID-19 vaccines in less than a year.
b. Production Scalability
- mRNA vaccine synthesis is entirely cell-free and amenable to scaled-up Good Manufacturing Practices (GMP) standards.
- It allows higher yields than traditional vaccines reliant on cultured cells or eggs.
c. Safety Profile
- mRNA vaccines do not use any form of the live virus, avoiding safety risks associated with weakened or inactivated pathogens.
- To date, mRNA vaccines have demonstrated excellent short-term safety data based on clinical trials and real-world monitoring.
d. High Efficacy
- mRNA vaccines for COVID-19 showed 94–95% efficacy in preventing symptomatic illness during trials.
- In real-world studies, effectiveness has remained high across age groups, preventing severe disease and death.
e. Adaptability
- The mRNA platform facilitates updating vaccines to match new viral strains if needed for variants.
- mRNA vaccines targeting multiple influenza strains or emerging viruses are in development.
The combination of speed, scalability, potency, and adaptability gives mRNA vaccines unmatched advantages that can revolutionize preparedness for emerging infectious threats.
Safety Testing and Approval Process for mRNA Vaccines
Some individuals have expressed concerns regarding the safety profile of these newly approved mRNA vaccines. However, it is essential to note that these vaccines have undergone large, careful clinical trials and comprehensive reviews by regulators across the globe.
- Clinical Trial Phases I-III: mRNA vaccines completed three stages of human trials with thousands of participants to assess safety, dosing, and efficacy.
- FDA Evaluation: mRNA vaccines were rigorously evaluated via the FDA’s emergency use authorization process and approved based on safety and efficacy data.
- External Expert Review: Authorization decisions incorporated input from independent advisory committees to analyze safety and effectiveness.
- Post-Approval Monitoring: Vaccine monitoring has continued after authorization to detect rare side effects not observed in clinical trials.
- Highly Transparent Process: Details of clinical trials, ingredient safety data, and ongoing monitoring are publicly shared in an unprecedented manner.
- Billions of Doses Administered: The excellent real-world safety record after billions of shots provides the most compelling evidence of vaccine safety.
This multilayered scrutiny and transparency should ensure that no shortcuts were taken in confirming the safety of mRNA vaccines through comprehensive, rigorous evaluation and review.
Evidence of mRNA Vaccine Effectiveness
Beyond controlled clinical trials, researchers have collected ample real-world data demonstrating the effectiveness of mRNA vaccines in preventing COVID-19 illness once rolled out globally.
- A Mayo Clinic study of 139,000 individuals found mRNA vaccine effectiveness of 96% against hospitalization and 92% against COVID-19 infection overall.
- A Kaiser Permanente study of 4 million members found mRNA vaccine effectiveness of 90% against SARS-CoV-2 infection and 96-97% against hospitalization.
- A CDC study of 10 million Americans showed unvaccinated individuals faced an 11-fold higher risk of death from COVID-19 than fully vaccinated individuals.
- Research across Mayo Clinic, Stanford, and VA facilities found mRNA vaccines to be over 90% effective at preventing infection from the Delta variant.
These extensive observational studies align with efficacy in clinical trials and demonstrate that mRNA vaccines prevent symptomatic cases, hospitalization, and death, including against Delta and other circulating variants.
Distribution Challenges for mRNA Vaccines
While mRNA vaccines have provided a hugely valuable tool to counter COVID-19, there have been hurdles regarding equitable distribution and access:
- Ultra-Cold Storage: mRNA vaccines require very cold temperatures for stability during transport and storage (-20°C for Moderna, -70°C for Pfizer). This dependence on specialized freezers creates logistical obstacles in the cold chain.
- Ramping Up Manufacturing: It took time to scale manufacturing capacity to produce billions of mRNA vaccine doses. Pfizer and Moderna struggled with early supply shortages.
- Resource Inequality: Wealthy nations claimed most of the mRNA vaccine supply early on, leaving developing regions vulnerable. It underscored global divides.
- Vaccine Hesitancy: Misinformation and mistrust of vaccines have fueled hesitancy among population segments, limiting uptake.
Despite remarkable efficacy, tangible barriers to access and acceptance have hindered the broader distribution and delivery of mRNA vaccines where they are most needed. Global collaboration and focused strategies will be vital in overcoming these challenges.
Potential Future Applications of mRNA Technology
While the COVID-19 vaccines made mRNA a household name, researchers see broad potential to apply this technology against other infectious diseases and conditions.
- Seasonal flu vaccines that can adapt to match circulating strains each year.
- Vaccines against high-risk pathogens like Ebola, Zika, HIV, and malaria
- Cancer treatment vaccines train the immune system against tumor proteins.
- Vaccines target the pathogens that cause rabies, shingles, and other common illnesses.
- Therapies use mRNA to replace defective genes or produce therapeutic proteins within the body.
A few mRNA vaccines and therapeutic candidates are already in various development and testing stages. Given the platform’s strengths, mRNA could provide ideal vaccines for diseases where traditional immunization strategies have fallen short. Ongoing innovation will help realize its full preventative and therapeutic potential.
Frequently Asked Questions about mRNA Vaccines
How do mRNA vaccines differ from conventional vaccines?
Unlike conventional vaccines that inject weakened or inactivated viruses to provoke immunity, mRNA vaccines deliver a synthetic mRNA sequence encoding instructions for cells to build harmless spike proteins that trigger an immune response. This approach does not use any form of live virus.
Can mRNA vaccines alter your DNA?
No. mRNA does not interact with or alter DNA. Once instructions to build viral proteins are completed, the mRNA breaks down in the cell, so it is not incorporated into human genes.
Why do mRNA vaccines require very cold temperatures?
To maintain stability, the mRNA compounds are fragile, so freezing storage at -20°C or below is needed during transport and storage. It requires special freezers. New formulations aim to improve temperature flexibility.
What are the main ingredients in mRNA vaccines?
The primary ingredients are the synthetic mRNA sequence plus a variety of lipids, salts, and stabilizers that form the protective lipid nanoparticles that deliver the mRNA into cells. Elements have been tested for safety.
How were mRNA vaccines developed so quickly for COVID-19?
Because mRNA vaccines are created directly from the viral genetic code, the sequence for the coronavirus spike protein was identified and synthesized into a vaccine very rapidly once the pandemic virus was genetically sequenced. It enabled unprecedented speed compared to more traditional methods.
Conclusion: mRNA Vaccines Are Pivoting Vaccine Development
The troubled COVID-19 pandemic has given rise to a breakthrough in vaccine technology. mRNA vaccines have proven their mettle against the novel coronavirus and appear poised to revolutionize infectious disease prevention programs in the future.
This guide provides a comprehensive overview of how mRNA vaccines work, their development, and their emerging benefits compared to conventional vaccines. While new mRNA vaccines have displayed remarkable effectiveness against COVID-19, no serious safety issues have been detected among the billions of doses administered. Their unique advantages can make mRNA vaccines ideal for combating fast-spreading or genetically prone pathogens.
Moving forward, mRNA vaccine technology will likely feature prominently in strategies to prepare for future pandemics. Their flexibility makes it feasible to develop new vaccines tailored to emerging viral threats rapidly. mRNA vaccines can also be harnessed to target stubborn infectious diseases like HIV, malaria, and cancer to begin making inroads against these intractable conditions.
The COVID-19 pandemic demanded unprecedented innovation, and mRNA vaccines rose to the challenge. Their success has opened the door to rethinking what’s possible for vaccine development and bringing new, effective vaccines to fruition faster than the decade-long timelines of the past. While work remains to improve global access and distribution, precise mRNA vaccine technology will transform immunization and usher in a new era of preparedness against infectious disease threats.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.