What are Acids and Bases? An acid (acid is a Latin word-“acidus” meaning “Sour”) was defined as a substance that its water solution tastes sour, turns blue litmus to red, and neutralizes bases soon. They have the following properties:
- They conduct the electricity in aqueous solutions
- They liberate hydrogen gas with active materials like Zinc, Magnesium, etc.
- They turn the blue litmus into the red.
- They are sour (sharp tasting) to taste.
A base was defined as a substance that its aqueous solution tastes bitter, turns red litmus to blue, neutralizes acids and so on. They have the following properties:
- They conduct electricity in aqueous solutions.
- They react with acids to neutralize them and form salts.
- They turn red litmus to blue.
- They are bitter to taste and their solutions are based on the experimental results.
Theories of Acids & Bases
There are three concepts of acids and bases in current use. They are based on the structure and composition of acids & bases. Three concepts are explained this acids & bases mechanism: they are,
- Arrhenius Theory
- Bronsted-Lowry Theory
- Lewis Theory
1) Arrhenius concept
Savante Arrhenius (1887) proposed his concept of acids and bases on his theory of electrolytic dissociation. He defined “Acids as those compounds which give H+ ions in aqueous solution. “All acidic properties are due to H+ ions. Bases are those compounds which furnish OH– ions in aqueous solution and all basic properties are due to these OH– ions”. For example,
HCl –> H+ + Cl–
(Aqueous) (Aqueous) (Aqueous)
NaOH –> Na+ + OH–
(Aqueous) (Aqueous) (Aqueous)
The strength of the acid (or) base, according to “Arrhenius concept” is based on their rates of ionization (or) equilibrium constants of acids and bases.
The undissociated acid (HX) is always in equilibrium with the ions.
HX –> H+ + X–
The equilibrium constant, Ka for the acid. HX is
[H] [X]
Ka = —————-
[HX]
Similarly, the equilibrium constant Kb for the base,
MOH –> M+ + OH–
[M+] [OH–]
Ka = ——————-
[MOH]
Limitations:
- This concept of acids and bases is limited to aqueous solutions only.
- It fails to explain the acidic character of CO2, SO2 and basic character of MgO and CaO.
- The neutralization process is limited to those reactions which can occur in aqueous solutions only, although reactions involving salt formation do occur in the absence of solvent.
- The hydration of hydrogen ion in aqueous solution was not explained.
Modified Arrhenius theory:
The theory extends even acid-base concept even to no aqueous solvents. It rectifies most of the defects. According to the modified concept, solvents also ionize to give positive and negative ions. For example,
H2O –> H+ + OH–
But the ions take up water become hydrated. Therefore the equation becomes,
H2O + H2O –> H3O+ (aqueous) + OH–(aqueous)
The substances, which increase the concentration of H3O+, hydronium ion, are acids and the hydroxyl ion (OH–) is bases.
- The attraction between H+ and OH– is strong.
- The site of a proton is comparable to that of its nucleus.
- So, unlike other ions, H+ ion can penetrate into the electron cloud of H2O and forms H3O.
- Usually, H3O combined with four to six water molecules.
- The decomposition of HCl in water cannot be written in the following way.
HCl –> H+ + Cl–
But it may be written as
HCl + H2O–> H3O+ (aqueous) + Cl–(aqueous)
2) Bronsted-Lowry concept
J.N.Bronsted and T.M.Lowry explain the concept of acids and bases in an attempt to rectify the limitations of Arheneous concept called “ Bronsted-Lowry acid-base theory”.
According to the concept, an acid is a substance that is capable of donating a proton (proton donor) while a base is a substance capable of accepting a proton (proton acceptor) from an acid. This theory is also known as “Proton theory”.
HCl acts an acid as it donates a proton to water molecules.
HCl + H2O –> H3O+ + Cl–
NH3 acts as a base it accepts a proton from water.
NH3 + H2O –> NH4+ + OH–
Loss of proton by acid results in the formation of a base, called “conjugative base”.
HA –> H+ + A–
(acid) (proton) (base)
A– also called conjugated base of acid HA.
Similarly, a base upon gaining a proton produces an acid, called “conjugate acid”.
H+ OH– –> H2O
H2O is called conjugate acid of base OH–. Thus every base has its conjugate acid and every acid has its conjugate base.
3) Lewis Concept
G.N.Lewis (1923) proposed the concept of acids and bases upon the electronic theory of valency to include those reactions where no proton transfer takes place.
According to this concept, an acid is an electron pair acceptor and a base is an electron-pair donor.
The formation of a coordinate bond by the sharing of an electron pair with acid by a base is called “neutralization”.
Example: Proton (H+) is a Lewis base as it can accept an electron pair. Ammonia (: NH3) has an electron pair which it can donate and this is a Lewis base.
Thus the Lewis reaction between H+ and NH3 can be written as:
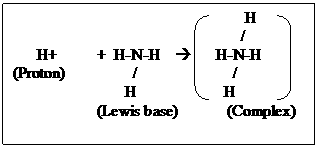
Limitations:
- It failed to explain the strength of acids & bases.
- Acids such as HCl, H2SO4 do not form coordinate covalent bonds with bases.
- Formation of a coordinate covalent bond is generally slow. Actually all acids-base reactions are very fast.
Lewis bases may be the following types:
- All simple ions such as Cl–, F– ions.
- Molecules with one (or) two unshared pair of electrons, E.g.: H2O, NH3, ROH, R2O, R2S, etc.
- Multiply bonded compounds which form coordination compounds with transition metals. E.g.: CO, NO, C2H4, C2H2, etc.
- All Lewis bases accept a proton by donating an electron pair to it. Hence all Lewis bases are “Bronsted bases”. Only some of the Lewis acids are proton donors, hence all Lewis acids are not bronsted acids.
Useful readings:
- What Are The Basics of pH? Do You Know The Importance?
- What are the Basic Color reactions of Amino acids?
- Physio Chemical Properties of Amino acids? (Guide)
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.