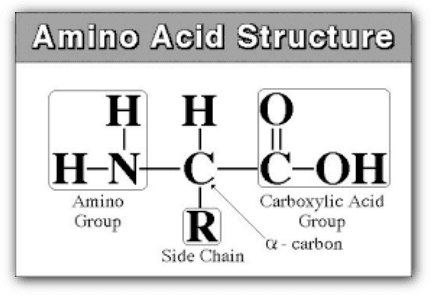
Amino acids are organic compounds that contain an amino group (-NH2) and a carboxyl group (-COOH) attached to a central carbon atom. They are the building blocks of proteins and perform various functions in living organisms.
There are about 500 amino acids known, but only 20 are encoded by the genetic code and used to synthesize proteins.
These 20 amino acids can be classified based on different criteria, such as the structure, polarity, charge, and function of their side chains (R groups).
This article will introduce the basic concepts of amino acid classification and its importance for understanding protein structure and function.
The proteins on hydrolysis yield mixtures of the component amino acids.
Therefore, to understand the structure and chemistry of proteins, we have to first undertake the study of amino acids.
Structure of Amino acid
Amino acids are the building blocks of proteins and have a common structure with some variations. The basic structure of an amino acid consists of a central carbon atom (called the alpha carbon) that is bonded to four different groups: a hydrogen atom, a carboxyl group (-COOH), an amino group (-NH2), and a variable group (called the R-group or side chain).
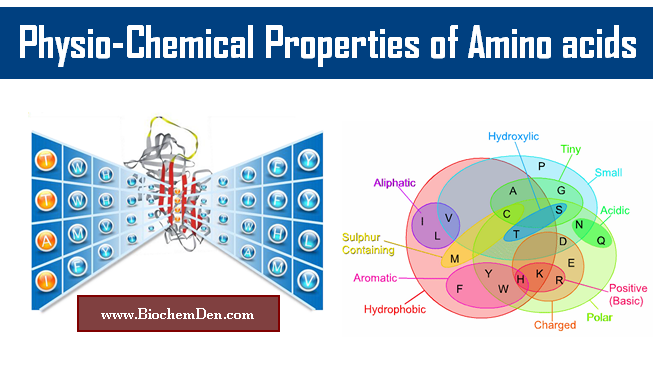
The R-group is what distinguishes one amino acid from another and determines its chemical properties. Amino acids can be classified into four categories based on their R-groups: nonpolar, polar, acidic, or basic.
- Nonpolar amino acids have hydrophobic R-groups that do not interact with water.
- Polar amino acids have hydrophilic R-groups that can form hydrogen bonds with water or other polar molecules.
- Acidic amino acids have R-groups that contain a carboxyl group (-COOH) that can donate a proton and become negatively charged.
- Basic amino acids have R-groups that contain an amino group (-NH2) that can accept a proton and become positively charged.
- Amino acids exist in a zwitterionic form, meaning they have both positive and negative charges on different atoms, at physiological pH.
Amino acids Classification
It can classify amino acids into 4 types:
- Classification based on the position of “-NH2”
- Classification based on the composition of the “-R’ side chain
- Classification based on the Nutritional requirement
- Classification based on the Metabolic Fate
1. Classification based on the position of “-NH2”
They can classify amino acids into THREE types:
i. α-amino acid
The amino group attached to the next carbon of the carboxyl group is called “α-amino acid” All naturally occurring amino acids are in “α-L-amino acids”.
ii. β-amino acid
The amino group attached to the third carbon (numbering from the Carboxyl group) of the amino acid is called “β-amino acid”. Eg: β-alanine, is one of the end products of Pyrimidine catabolism.
iii) γ-amino acid
The amino group attached to the Fourth carbon (numbering from the Carboxyl group) of the amino acid is called “γ-amino acid”. Eg: GABA (Gamma Amino Butyric Acid)
2. Classification based on the composition of “-R’ side chain
Based on the composition of the ‘R’ side chain, amino acids can be categorized into 8 types: (Fairly & Kigour, 1966)
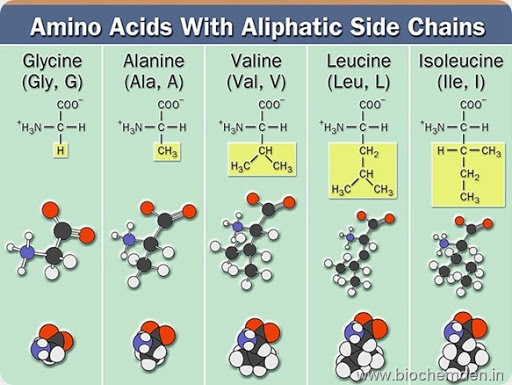
a) Neutral Amino Acids (or) Simple amino acids
These have no functional group in the side chain. Eg:
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Glycine (glycosG=sweet) | G | Gly | α-amino acetate | Animal sources are Scleroproteins, Gelatin, and silk fibroin. Plant sources are Glycine Max (Soya been) |
| Alanine (Isolated from Silk Fibroin in 1888.) | A | Ala | α-amino propionate | Alanine is present in Silk fibroin along with Glycine |
| Valine | V | Val | α-amino isovalerate | |
| Leucine (Isolated from Cheese by Proust in 1819.) | L | Leu | α-aminoisocaproate | Isolated from cheese, but later it was obtained in purer form from hydrolysates of wool. |
| Isoleucine (Discovered by Paul Erhlish (LT 1854 to 1915)) | I | Ile | α-amino-β-methylvalarate |
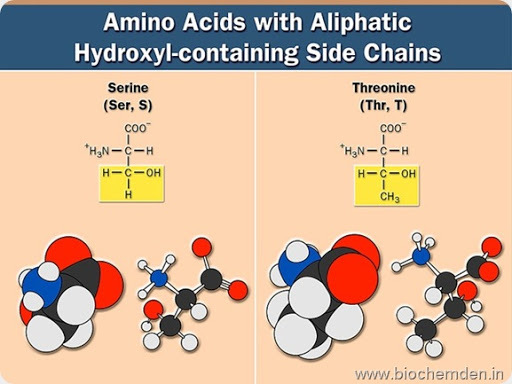
b) Hydroxyl Group containing amino acids
These contain a hydroxyl group in their side chain. Eg:
| Name of the Amino acid | Single letter symbol | Three letter symbol | IUPAC name | Source |
| Serine (Derived from the Serum) | S | Ser | α-amino-β-hydroxyl propionate | Silk protein, Sericin, and Fibroin |
| Threonine (Discovered by Meyer & Rose in 1936) | T | Thr | α-amino-β-hydroxyl butyrate | Threonine is less abundant than serine in most proteins. |
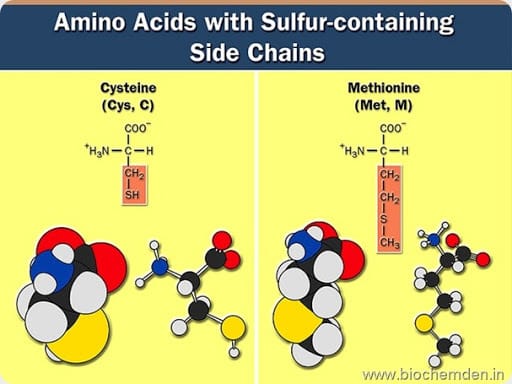
c) Sulphur Containing amino acids
These possess a sulfur atom in the side chain. Eg:
| Name of the Amino acid | Single letter symbol | Three letter symbol | IUPAC name | Source |
| Cysteine (Isolated from Urinary stones in 1843) | C | Cys | α-amino-β-mercaptopropionate | Fibrous proteins such as Keratin from hair are especially rich in cysteine (12%) |
| Methionine | M | Met | α-amino-β-methylmercaptobutyrate |
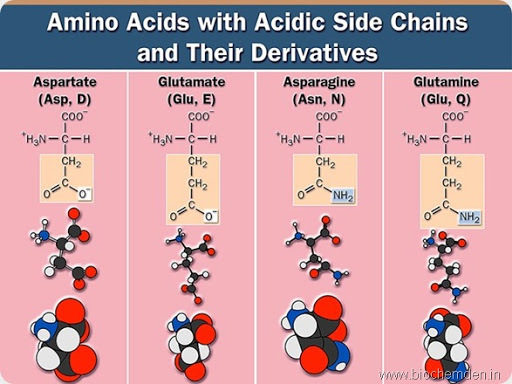
d) Acidic amino acids
These have a Carboxyl group in the side chain
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Aspartic acid (Discovered by Ritthausen in 1868) | D | Asp | α-amino glutarate | It is the parent compound of aspargine. |
| Glutamic acid (Discovered by Ritthausen in 1866) | E | Glu | α-aminoglutarate | It is found in Gluten. It is the parent compound of Glutamine. |
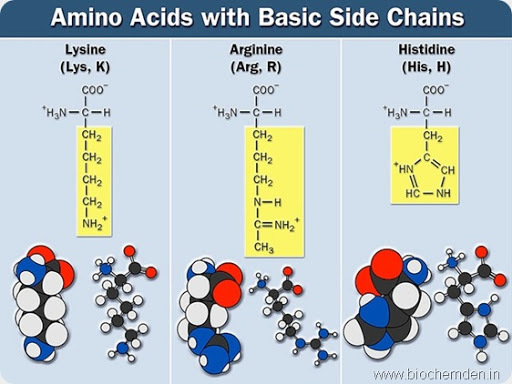
e) Basic amino acids
These possess an amino group in the side chain. Eg:
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Arginine | R | Arg | α- amino-δ-guanidinovalarate(Guanidonium group is present) | It is abundant in highly basic proteins of the cell nucleus (histones) and in Sperm proteins. |
| Lysine | K | Lys | α, ε- diaminocaproate | It is present in plant proteins like Corn and Wheat. |
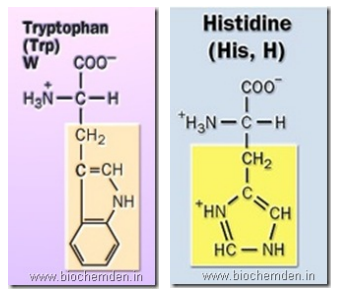
f) Heterocyclic amino acid
These amino acids have in their side chain a ring that possesses at least one atom other than carbon. Eg:
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Tryptophan (It was discovered in the laboratory of F.G.Hopkins) | W | Trp | α-amino-β—3-indolepropionate (or) β–indolylalanine | |
| Histidine | H | His | α-amino-β-Imidazolepropionate | Hemoglobin, Protamines and Histones |
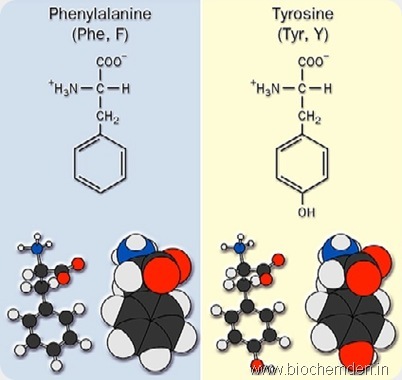
g) Aromatic amino acid
These have a benzene ring in the side chain. Eg:
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Phenylalanine | F | Phe | α-amino-β-phenylpropionate | |
| Tyrosine (Isolated from Cheese in 1857) | Y | Tyr | α-amino-β-(p-hydroxy phenyl) propionate | Cheese |
h) Imino acid
These are also heterocyclic compounds, which have an “imino group” (-NH-) instead of an amino group (-NH2).
| Name of the Amino acid | Single letter symbol | Three letters symbol | IUPAC name | Source |
| Proline | P | Phe | 2-pyrrolidinecarboxylate | Zein from Corn and Gelatin |
| Hydroxy Proline | – | Hy.Pro |
3. Classification based on the Nutritional requirement
Based on Nutritional requirements, amino acids can be divided into 3 types.
- Essential Amino acids (EAA)
- Non-Essential Amino acids (NEAA)
- Semi-Essential Amino acids (SEAA)
1. Essential Amino acids (EAA)
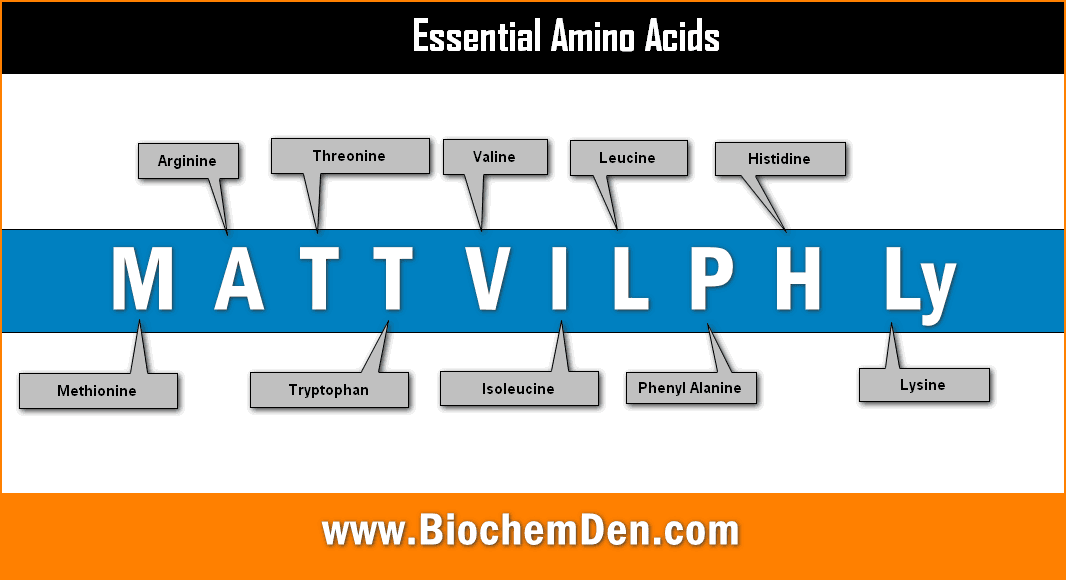
Some amino acids don’t synthesize in the human body. It should be supplied through diet. They are required for the proper growth and maintenance of the individual.
Eg:
MATT VIL PHLy
(or)
PVT TIM HALL
M= Methionine A=Arginine T=Threonine T=Tryptophan V=Valine
I=Isoleucine L=Leucine P=Phenylalanine H=Histidine L=Lysine
2. Non-Essential Amino acids (NEAA)
The body can synthesize about 10 amino acids to meet biological needs, hence they need not be consumed in the diet.
Eg: Gly, Ala, Ser, Cys, Asp, Asn, Glu, Gln, Tyr, and Pro.
3. Semi-Essential Amino acid
Histidine and Arginine are semi-essential amino acids. Growing children require them in food. But they are not essential for the adult individual.
4. Classification based on the Metabolic Fates
In this Amino acids classification, it can be classified based on the basis of metabolic fate:
- Purely ketogenic amino acids
- Ketogenic and Glucogenic amino acids
- Purely Glucogenic amino acids
i. Purely ketogenic amino acids
Leucine is purely ketogenic because it is converted into ketone bodies.
ii. Ketogenic and Glucogenic amino acids
During metabolism, part of the carbon skeleton of these amino acids will enter the ketogenic pathway and the other part of the glucogenic pathway.
Eg: Lys, Ile, Phe, Tyr & Trp are partially ketogenic and partially glucogenic.
iii. Purely Glucogenic amino acids
All the remaining 14 amino acids are purely glucogenic as they enter only into the glucogenic pathway.
Reference: Structural Amino acids
Frequently Asked Questions (FAQs)
What is an amino acid?
Amino acids are the building blocks of proteins. They are organic compounds made up of an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a side chain (also known as an R-group).
What are the four types of amino acids?
There are four types of amino acids, which are categorized based on the chemical properties of their side chains. They are:
1. Non-polar amino acids
2. Polar amino acids
3. Acidic amino acids
4. Basic amino acids
What are the structural properties of amino acids?
Amino acids have a central carbon atom (called the alpha carbon) bonded to four groups: the amino group (-NH2), the carboxyl group (-COOH), a hydrogen atom, and a side chain (R-group). The R-group determines the chemical properties of the amino acid.
How many amino acids are in a protein?
The number of amino acids in a protein depends on the protein’s size and function. Proteins are made up of long chains of amino acids, and the average protein has between 100 and 1,000 AAs.
How to memorize 20 amino acid names?
There are several methods to memorize the 20 amino acid names, including using flashcards, creating mnemonic devices, and practicing repetition. One popular mnemonic device is “Private Tim Hall Always Has His Army In Socks,” which stands for the first letter of each amino acid name.
Additional readings:
- Physio-Chemical Properties of Amino Acids? (Guide)
- What is Amino acid and its Structural Chemistry?
- What are the Fundamental molecules of Proteins?
Conclusion
Amino acids are the basic building blocks of proteins and have various structures and functions. It can be classified based on the location of their functional groups, their polarity, their ionization, their side chain group type, their distribution in proteins, and their nutritional requirements.
Amino acids play important roles in biological processes such as metabolism, gene expression, enzyme catalysis, and signaling. These are essential nutrients for humans and other organisms and must be obtained from dietary sources or synthesized by the body.
Amino acids are also involved in many industrial applications such as food additives, pharmaceuticals, cosmetics, and biotechnology. So these are fascinating molecules that deserve further study and exploration.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.