In biochemistry, the biosynthesis of eicosanoids represents a complex enzymatic process where 20-carbon polyunsaturated fatty acids are transformed into potent lipid mediators. These Molecules regulate critical physiological functions, including
- Growth and recovery during and after physical activity
- Inflammatory response and immune function following pathogenic exposure
- Neurotransmission and signaling in the central nervous system
- Vascular tone regulation and hemostasis
The biosynthesis of eicosanoids is initiated through the release of arachidonic acid (AA) from membrane phospholipids, followed by enzymatic conversion through three major pathways:
- cyclooxygenase (COX),
- lipoxygenase (LOX) and
- cytochrome P450-mediated pathways
This cascade produces diverse eicosanoid families, including prostaglandins, leukotrienes, and epoxyeicosatrienoic acids (EETs), each with distinct physiological roles.
| Pathway | Key Enzyme | Primary Products | Physiological Role |
|---|---|---|---|
| COX | Cyclooxygenase (COX-1, COX-2) | Prostaglandins (PGE₂, PGI₂), Thromboxane A₂ (TXA₂) | Inflammation, pain signaling, platelet aggregation, hemostasis |
| 5-LOX | 5-Lipoxygenase | Leukotrienes (LTB₄, LTC₄, LTD₄) | Immune cell recruitment, bronchoconstriction, airway tone |
| 12-LOX | 12-Lipoxygenase | 12-HETE, Lipoxins | Anti-inflammatory signaling, resolution of inflammation |
| CYP | Cytochrome P450 epoxygenases | EETs, 20-HETE | Vascular tone regulation, renal function, blood pressure control |
Release of Arachidonic Acid
The first step in the biosynthesis of eicosanoids is liberation of arachidonic acid (AA) from membrane phospholipids by phospholipases.
- Cytosolic phospholipase A2 (cPLA2) is the dominant enzyme, activated by increased intracellular Ca²⁺ and phosphorylation, and it hydrolyzes the sn‑2 position of phospholipids to release AA.
- Alternatively, phospholipase C (PLC) can generate diacylglycerol (DAG), which is further cleaved by DAG lipase to yield free arachidonic acid that enters eicosanoid biosynthetic pathways.
Nuclear and endoplasmic reticulum membranes are considered major reservoirs of esterified arachidonic acid for eicosanoid formation.
Major Enzymatic Pathways in Eicosanoid Biosynthesis
Once arachidonic acid is released, it can be metabolized through three main pathways that define the biosynthesis of eicosanoids.
- Cyclooxygenase (COX) pathway: Produces prostaglandins, prostacyclin, and thromboxanes.
- Lipoxygenase (LOX) pathway: Generates leukotrienes, hydroxyeicosatetraenoic acids (HETEs), and lipoxins.
- Cytochrome P450 (CYP) pathway: Forms epoxyeicosatrienoic acids (EETs) and additional HETEs involved in vascular and renal regulation.
These pathways operate in parallel and are tightly regulated at the level of enzyme expression, substrate availability, and feedback from downstream mediators.
How Eicosanoids Are Synthesized in the Human Body:

Figure 1.1-1:
a) Alternate pathways of Arachidonic acid release
b) cellular locations of enzymes involved in Eicosanoid formation
- a: Arachidonic acid may be directly released by phospholipase A2 (PLA2), or alternatively by the successive action of phospholipase C (PLC) and diacylglycerol (DAG) lipase.
- b: The major mechanism of release involves a cytosolic phospholipase A2 (cPLA2). An increase of Ca++ in response to an extrinsic signal causes binding of cPLA2 to the nuclear membrane. Cyclooxygenase (COX) and lipoxygenase (LOX) form their respective intermediates, which are further processed by cytosolic enzymes to prostaglandins (PG), thromboxanes (TG), and leukotrienes (LT), respectively.
The major physiological mechanism of release consists in the activation of a cytosolic phospholipase A2 (cPLA2) by Ca++ in response to an extracellular signal. cPLA2 then attaches to the nuclear (and probably ER) membranes, which appear to be the major reservoir of arachidonic acid and its analogs.
- What are lipids? What is its importance in the human body?
- Lipid Metabolism: Simple Smart Guide and Notes
The formation of the most important eicosanoid derivatives of arachidonic acid and its analogs is initiated by cyclooxygenases (Cox) and lipoxygenases (Lox):
- Cyclooxygenase, also called prostaglandin H synthase, converts arachidonic acid first into prostaglandin G2 (PGG2) and then PGH2. PGH2 is the common precursor of prostaglandins E2 and F2 and of prostaglandin I2 (prostacyclin). It is also the precursor of thromboxane A2 (TXA2). Therefore, cyclooxygenase is the single most important enzyme and drug target in eicosanoid metabolism.
- Lipoxygenase 5 converts arachidonic acid into 5-hydroperoxy-eicosatetraenoic acid (5-HPETE), which is the precursor of leukotrienes. Leukotrienes are formed mainly in leukocytes, e.g., macrophages and granulocytes, and they are potent pro-inflammatory mediators. Suppression of leukotriene synthesis with inhibitors of lipoxygenases is a fairly recent therapeutic principle in the treatment of asthma and chronic inflammation.
- 12- and 15-HPETE are formed by the corresponding lipoxygenases 12 and 15. They can be reduced to hydroxy-eicosatetraenoic acids (HETEs) and further converted to lipoxins (cf. Figure 1.2-5). They have their own receptors and physiological roles, but they are not presently in the focus of interest of drug therapy or development.
Figure 1.2-5:
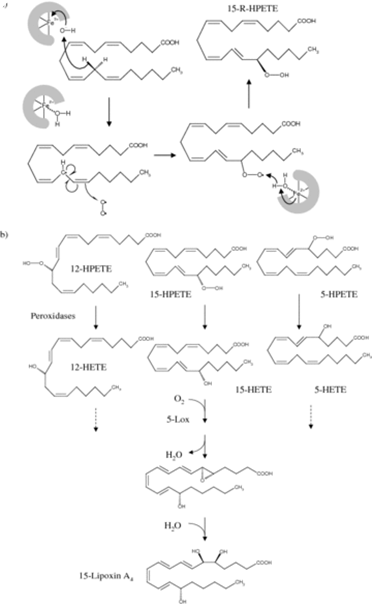
- Eicosanoids are derived by lipoxygenases. a: Mechanism of reaction, illustrated for 15-lipoxygenase.
- Abstraction of a hydrogen atom by a non-heme iron in the active site leads to a carbon radical intermediate, which subsequently reacts with an oxygen π radical.
- b: Products of the three lipoxygenases (5-, 12-, and 15-lipoxygenase).
- The initial hydroperoxy products (HPETE = hydroperoxyeicosatetraenoic acid) are reduced by glutathione peroxidases to hydroxy derivatives (HETE). HETEs may have signalling roles of their own, and they may also be converted further to lipoxins.
- This conversion is initiated by another lipoxygenase, as shown here for 5-lipoxygenase (5-Lox) acting on 15-HETE. 5-HPETE is also the precursor of the leukotrienes.
The major ‘classical’ drug target in prostaglandin metabolism is cyclooxygenase, which occurs in several isoforms: Cox-1, Cox-2, and apparently in some mammals Cox-3. This is due to the central role of its product prostaglandin H2 as a precursor of multiple eicosanoid mediators (Figure 1.1-3).
Figure 1.1-3:

Structures of prostaglandin H2 and its derivatives. PG, prostaglandin; TX, thromboxane.
The synthesis of PGH2 occurs in two separate successive reactions (Figure 1.1-2), for which there are two separate active sites on the cyclooxygenase molecule.
- Essential Fatty Acids Definition and Notes in Biology
- Fatty Acid Synthesis: Activation, Steps and Control
Figure 1.1-2:

The two reactions are catalyzed by cyclooxygenase. For each of these reactions, there is a separate active site.
The first reaction—also referred to as the cyclooxygenase reaction—introduces two peroxy groups, one endoperoxide (forming a ring with carbons 9-11) and a hydroperoxide attached to position 15.
The resulting product—prostaglandin G2—is quite unstable, yet it is able to dissociate from the enzyme and to rebind to the second active site of the same or another enzyme molecule.
There, the hydroperoxide is reduced to a simple hydroxy group, at the expense of two equivalents of reduced glutathione. This second step is called the peroxidase reaction.
Figure 1.1-4:

Spatial relationship of the two active sites in cyclooxygenase 1.
- a: Heme (green, with iron in magenta) and arachidonic acid (white, space-filling) bound within the peroxidase and the cyclooxygenase sites, respectively.
- b: Location of three important amino acid residues within the cyclooxygenase active site. Note that Tyr 385 is positioned between the two active sites.
- c: Space-filling representation of arachidonic acid (yellow), Tyr 385 (white), and heme (green).
The two active sites of cyclooxygenase are located close to each other (Figure 1.1-4a), and it is believed that this proximity is important in the ‘priming’ of the cyclooxygenase site.
The first step in the cyclooxygenase reaction (Figure 1.1-5) is initiated by a tyrosyl radical (Tyr385 in cyclooxygenase 1; Figure 1.1-4b,c).
Figure 1.1-5:

- Catalytic mechanism of the cyclooxygenase reaction, leading from arachidonic acid (top left) to prostaglandin G2 (top right). Y· and YH represent Tyr 385.
- Molecular oxygen reacts in its π-radical form.
- Note that this is only the first one of the two reactions catalyzed by cyclooxygenase.
This tyrosyl radical will not exist in a newly translated enzyme molecule, and once it is there, it may be lost due to capture of a hydrogen from somewhere else than the substrate. There thus has to be a mechanism for its formation or regeneration. This mechanism is provided by the heme in the peroxidase active site.
- Hemoglobin: Structure, Function and its Properties
- Glycogenolysis: How Glycogen Is Utilized in Animals
The heme radical cation, which forms as an intermediate during the peroxidase reaction, can abstract a hydrogen atom from the tyrosine -OH group, which thus may act as a reductant in place of one of the glutathione molecules normally functioning as cosubstrates (Figure 1.1-6b).
Figure 1.1-6:

- Overview of the peroxidase reaction of cyclooxygenase. a: Prostaglandin G2 enters the peroxidase active site after leaving the cyclooxygenase site.
- It is reduced by heme, which thereby is converted into the cation radical form (red).
- The heme is reduced in turn by glutathione (GSH) in a stepwise fashion. b: ‘Priming’ of the cyclooxygenase site.
- The heme cation radical abstracts a hydrogen atom from Tyr 385, which thereby replaces one glutathione.
- The prostaglandin G (PGG) that is required to form the heme cation radical in the first place must be provided by another enzyme molecule. (AA-H: arachidonic acid)
The peroxidase reaction (Figure 1.1-6a) can function in the absence of cyclooxygenase activity because the intermediate product (prostaglandin G2) can be provided by another enzyme molecule.
Cyclooxygenase Pathway: Prostaglandins and Thromboxanes
The cyclooxygenase pathway in eicosanoid biosynthesis is catalyzed by prostaglandin endoperoxide synthase (COX‑1 and COX‑2).
- COX converts arachidonic acid first to prostaglandin G2 (PGG2) and then to prostaglandin H2 (PGH2) via coupled cyclooxygenase and peroxidase activities.
- PGH2 serves as the common precursor for several eicosanoids: prostaglandin E2 (PGE2), PGF2α, prostacyclin (PGI2), and thromboxane A2 (TXA2), each produced by tissue‑specific synthases.
PGI2 is a vasodilator and inhibitor of platelet aggregation, whereas TXA2 promotes vasoconstriction and platelet aggregation, highlighting the opposing physiological roles within the biosynthesis of eicosanoids network.
Lipoxygenase Pathway: Leukotrienes, HETEs, and Lipoxins
The lipoxygenase pathway of eicosanoid biosynthesis introduces molecular oxygen into arachidonic acid to form hydroperoxy intermediates.
- 5‑lipoxygenase (5‑LOX) converts arachidonic acid into 5‑HPETE, which is then processed into leukotriene A4 (LTA4) and further to leukotrienes such as LTB4, LTC4, LTD4, and LTE4, potent mediators of inflammation and bronchoconstriction.
- 12‑LOX and 15‑LOX yield 12‑HPETE and 15‑HPETE, which are reduced to HETEs and can be further metabolized into lipoxins that generally exert anti‑inflammatory and pro‑resolving effects.
Leukotrienes are synthesized mainly in leukocytes like neutrophils, eosinophils, and macrophages and play a central role in asthma and allergic inflammation.
Cytochrome P450 Pathway in Eicosanoid Biosynthesis
The cytochrome P450 (CYP) pathway contributes additional branches to the biosynthesis of eicosanoids by epoxidation and hydroxylation of arachidonic acid.
- CYP epoxygenases generate epoxyeicosatrienoic acids (EETs), which modulate vascular tone, endothelial function, and renal sodium handling.
- CYP ω‑hydroxylases produce 20‑HETE and related metabolites that influence vasoconstriction, tubular transport, and blood pressure control.
Although less emphasized in classic textbooks, CYP‑derived eicosanoids are increasingly recognized as important regulators in cardiovascular and renal physiology.
Transcellular Biosynthesis of Eicosanoids
In many tissues, complete biosynthesis of eicosanoids requires cooperation between different cell types, a process termed transcellular biosynthesis.
- A donor cell possessing primary enzymes (e.g., COX or LOX) generates unstable intermediates such as LTA4 or PGH2 and releases them into the extracellular space.
- Neighboring acceptor cells, which may lack the initial enzyme but express specific downstream synthases, then convert these intermediates into biologically active eicosanoids.
This transcellular metabolism expands the repertoire of eicosanoids produced within a tissue and allows fine‑tuned local regulation.
Regulation and Clinical Relevance
The biosynthesis of eicosanoids is tightly controlled at several levels, including precursor availability, phospholipase activation, and inducible expression of COX‑2 and certain LOX isoforms during inflammation.
- Nonsteroidal anti‑inflammatory drugs (NSAIDs) inhibit COX enzymes, thereby reducing prostaglandin and thromboxane synthesis and alleviating pain, fever, and inflammation.
- Selective 5‑LOX inhibitors and leukotriene receptor antagonists are used in the management of asthma by limiting leukotriene production or action.
Dysregulated eicosanoid biosynthesis is implicated in cardiovascular disease, asthma, arthritis, cancer, and metabolic disorders, making these pathways important therapeutic targets.
On-page SEO suggestions (for your implementation)
- Update SEO title to: “Biosynthesis of Eicosanoids: Pathways, Enzymes and Functions”.
- Meta description example: “Learn the biosynthesis of eicosanoids from arachidonic acid: COX, LOX, and CYP pathways, key enzymes, intermediates, and clinical relevance in inflammation and disease.”
- Add the primary keyword “biosynthesis of eicosanoids” in: H1, first 100 words, 1–2 H2s, image alt text, and conclusion paragraph, while keeping density around 1–1.5%.
Key Enzymes in Eicosanoid Biosynthesis
Cyclooxygenase (COX)
Prostaglandin H synthase catalyzes the first committed step in the biosynthesis of eicosanoids
through the COX pathway. This enzyme exists in two main isoforms:
- COX-1: Constitutively expressed; produces basal eicosanoid levels
- COX-2: Inducible; upregulated during inflammation and stress
Both isoforms convert arachidonic acid to PGH₂, the common precursor for multiple eicosanoid
mediators. NSAIDs target COX enzymes, effectively reducing the biosynthesis of eicosanoids
derived from this pathway.
Lipoxygenase (LOX)
Three main lipoxygenase isoforms contribute distinct branches to eicosanoid biosynthesis:
- 5-LOX: Generates leukotrienes (LTA₄, LTB₄, LTC₄, LTD₄, LTE₄)
- 12-LOX: Produces 12-HETE and contributes to lipoxin formation
- 15-LOX: Forms 15-HETE and lipoxins with pro-resolving properties
Leukotrienes are particularly important in the biosynthesis of eicosanoids related to
allergic responses and asthma pathophysiology.
In accord with this model, a sample of cyclooxygenase, when expressed recombinantly and in the absence of arachidonic acid substrate, will initially be inactive, but it will exhibit ‘burst’ kinetics upon first contact with arachidonic acid, due to the cascading activation of more and more enzyme molecules by PGG2.
Regulation of Eicosanoid Biosynthesis
The biosynthesis of eicosanoids is tightly regulated at multiple levels:
1. Substrate Availability
- Arachidonic acid release depends on intracellular Ca²⁺ concentration
- Phospholipase A2 (PLA2) activation represents the rate-limiting step
- Feedback inhibition by some eicosanoids suppresses further biosynthesis
2. Enzymatic Regulation
- COX-1 is constitutive; COX-2 is inducible by inflammatory signals
- Competitive inhibition between LOX and COX pathways for substrate
- Transcriptional upregulation during immune activation and stress
3. Transcellular Biosynthesis
Different cell types specialize in different steps of the biosynthesis of eicosanoids:
- Mast cells, macrophages → initiate with 5-LOX or COX
- Platelets → complete TXA₂ synthesis
- Endothelial cells → generate PGI₂
- Neutrophils → produce leukotrienes
This cooperation allows tissue-specific eicosanoid production and amplifies local responses.
Clinical Applications of Targeting Eicosanoid Biosynthesis
Anti-inflammatory Therapies
NSAIDs reduce the biosynthesis of eicosanoids by inhibiting COX enzymes. Common examples include:
- Ibuprofen, naproxen (non-selective COX inhibitors)
- Celecoxib (selective COX-2 inhibitor)
Asthma and Allergic Conditions
Targeting the biosynthesis of eicosanoids through LOX inhibition:
- 5-LOX inhibitors: Zileuton
- Leukotriene receptor antagonists: Montelukast, zafirlukast
Cardiovascular Protection
Modulating the biosynthesis of eicosanoids to promote beneficial mediators:
- Low-dose aspirin shifts prostaglandin balance toward protective PGI₂
- EETs show promise in hypertension management (CYP-derived eicosanoids)
Emerging Targets
Research into the biosynthesis of eicosanoids increasingly focuses on:
- Lipoxin augmentation for resolving inflammation
- CYP-epoxygenase activation for vascular health
- Selective 5-LOX modulation for autoimmune conditions
Frequently Asked Questions About Eicosanoid Biosynthesis
What is the biosynthesis of eicosanoids?
The biosynthesis of eicosanoids is the enzymatic process that converts arachidonic acid and other 20-carbon fatty acids into bioactive lipid mediators through COX, LOX, and CYP pathways.
Which enzyme initiates the biosynthesis of eicosanoids?
Cytosolic phospholipase A2 (cPLA2) releases arachidonic acid from membrane phospholipids, which is the critical first step.
How does the biosynthesis of eicosanoids relate to inflammation?
Many eicosanoids produced during biosynthesis (prostaglandins, leukotrienes) are pro-inflammatory mediators that promote immune response and vascular permeability.
What drugs target the biosynthesis of eicosanoids?
NSAIDs (ibuprofen, naproxen) inhibit COX-mediated biosynthesis, while leukotriene antagonists target LOX-mediated biosynthesis in asthma treatment.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.