Carbohydrates have the general formula [CH2O]n where n is a number between 3 and 6. Carbohydrates function in short-term energy storage (such as sugar); as intermediate-term energy storage (starch for plants and glycogen for animals); and as structural components in cells (cellulose in the cell walls of plants and many protists), and chitin in the exoskeleton of insects and other arthropods.
Sugars are structurally the simplest carbohydrates. They are the structural unit that makes up the other types of carbohydrates.
Basic Overview of Classification of Carbohydrates
1. Monosaccharides
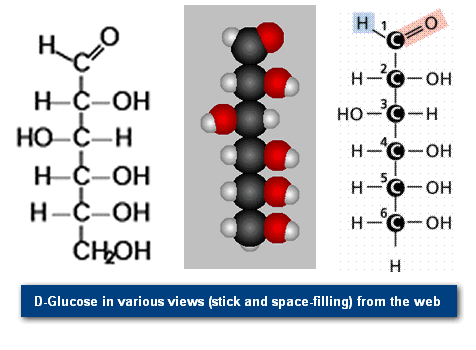
Monosaccharides are single (mono=one) sugars. Important monosaccharides include ribose (C5H10O5), glucose (C6H12O6), and fructose (same formula but different structure than glucose).
We classify monosaccharides by the number of carbon atoms and the types of functional groups present in the sugar. For example, glucose and fructose, illustrated in Figure 2, have the same chemical formula (C6H12O6), but a different structure: glucose having an aldehyde (internal hydroxyl shown as: -OH) and fructose having a keto group (internal double-bond O, shown as =O).
This functional group difference, as small as it seems, accounts for the greater sweetness of fructose as compared to glucose.
- In an aqueous solution, glucose tends to have two structures, α (alpha) and β (beta), with an intermediate straight-chain form (shown in Figure 3).
- The a-form and b- form differ in the location of one -OH group, as shown in Figure 2a.
- Glucose is a common hexose, a six-carbon sugar, in plants.
- The products of photosynthesis are assembled to form glucose. Energy from sunlight is converted into and stored as C-C covalent bond energy.
- This energy is released in living organisms in such a way that not enough heat is generated at once to incinerate the organisms.
- One mole of glucose yields 673 Kcal of energy. (A calorie is the amount of heat needed to raise one gram of water one degree C. A Kcal has 1000 times as much energy as a cal.). Glucose is also the form of sugar measured in the human bloodstream.
2.Disaccharides
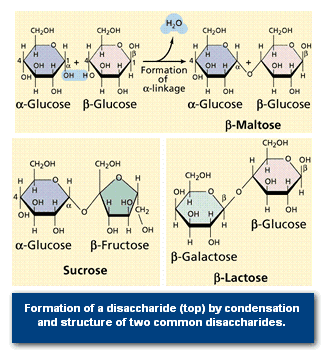
Disaccharides are formed when two monosaccharides are chemically bonded together. Sucrose, a common plant disaccharide is composed of the monosaccharides glucose and fructose. Lactose, milk sugar, is a disaccharide composed of glucose and the monosaccharide galactose.
The maltose that flavours a malted milkshake (and other items) is also a disaccharide made of two glucose molecules bonded together as shown in Figure 4.
3. Polysaccharides
Polysaccharides are large molecules composed of individual monosaccharide units. A common plant polysaccharide is a starch, which is made up of many glucose molecules (in a polypeptide these are referred to as Glucans). Two forms of polysaccharides – amylose and amylopectin make up what we commonly call starch.
The formation of the ester bond by condensation (the removal of water from a molecule) allows the linking of monosaccharides into disaccharides and polysaccharides. Glycogen (see Figure 5) is an animal storage product that accumulates in the vertebrate liver.
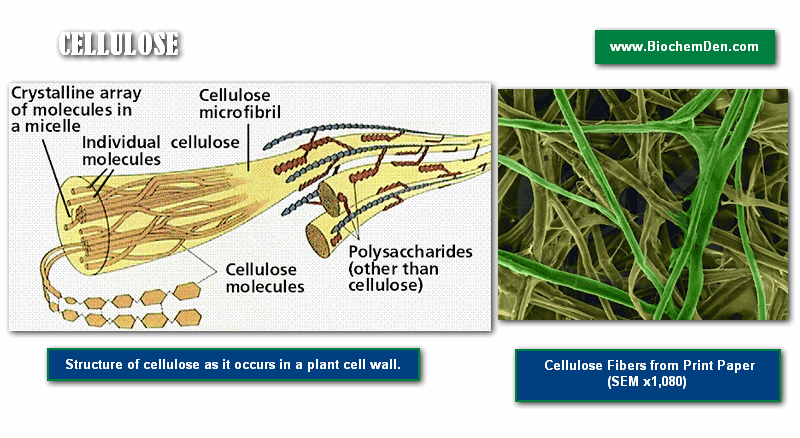
Cellulose
Cellulose, illustrated in Figures 6 and 7, is a polysaccharide found in plant cell walls. Cellulose forms the fibrous part of the plant cell wall. In terms of human diets, cellulose is indigestible, and thus forms an important, easily obtained part of dietary fiber. As compared to starch and glycogen, which are each made up of mixtures of ‘a’ and ‘b’- glucose, cellulose (and the animal structural polysaccharide chitin) are made up of only b-glucose.
The three-dimensional structure of these polysaccharides is thus constrained into straight microfibrils by the uniform nature of the glucose molecules, which resist the actions of enzymes (such as amylase) that break down storage polysaccharides (such a starch).
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.