There are five different kinds of electron carriers that participate in the transport of electrons from substrates as they are oxidized in the mitochondria. In addition, Cu+2 is present and functions in the enzyme, Cytochrome oxidase, that catalyzes the reduction of O2.
Mitochondrial Electron Transport Chain components:
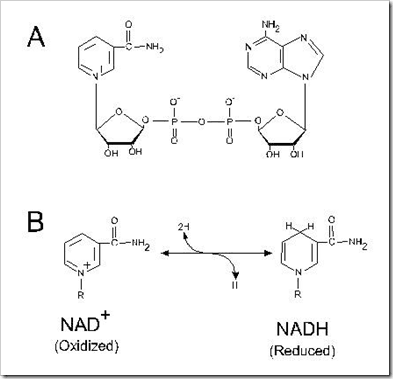
(1) Nicotinamide Nucleotides:
Two of the oxidation in the TCA Cycle involve the removal of the equivalent of two hydrogen atoms from the substrates, malate and isocitrate. In two others, those catalyzed by pyruvate dehydrogenase and α-Ketogularate dehydrogenase, the electrons are transferred first to lipoic acid and then via a flavorprotein to NAD+.
SH2 + NAD+ –> S + NADH + H+
(2) Flavoproteins:
These proteins contain a very tightly, sometimes covalently bound flavin nucleotide, either FMN (or) FAD. The oxidized flavin nucleotide can accept either one electron (or) two (yielding FADH2 (or) FMNH2). The standard reduction potential of a flavin nucleotide, unlike that of NAD (or) NADP, depends on the protein with which it is associated. The flavin nucleotide should be considered part of the flavoproteins active site, not as a resultant (or) product6 in the electron-transfer reaction. Because flavoproteins can participate in either one-or-two electron transfers, they can serve as intermediate between reactions in which two electrons are donated and these in which only one electron is accepted.
NADH + H+ + FMN —> NAD+ + FMNH2
Succinate + FAD –> Fumarate + FADH2

(3) Iron – Sulfur proteins:
- This type of protein was first encountered as ferredoxin, a reducing agent involved in nitrogen fixation and photosynthesis in plants before it was recognized to function in mt.E.T in animals.
- The iron atoms are arranged in pairs in an iron-sulfur bridge, which is bounded to the sulfur atoms of Cysteine residues in the protein.
- Some iron-Sulfur proteins such as spinach ferredoxin contains only two iron atoms (Fe2S2) while others contain four (Fe4S4)
(4) Quinones:
Mitochondria contain quinine called “Ubiquinone” (also called “Coenzyme.Q” (or) simply “Q”) which is a benzoquinone with a long isoprenoid side chain. Ubiquinone can accept one electron to become the semiquinone radical (QH*) or two electrons to form ubiquinol (QH2), it can act at the junction between a two electron donor and a one-electron acceptor, because ubiquinone is both small and hydrophobic, it is finally diffusible within the lipid bilayer of the inner mitochondrial membrane and can shuttle reducing equivalents between other, less mobile electron carriers in the membrane and because it carries both electrons and protons, it plays a central role coupling in coupling electron flow to proton movement.
(5) Cytochromes:
The cytochromes are proteins with characteristic strong absorption of visible light, due to their iron-containing heme prosthetic groups. Mitochondria consists of three classes of cytochromes, designated “Cyt.a ,Cyt.b and Cyt.c” distinguished by differences in their light-absorption spectra. Each type of cytochromes in its reduced (Fe2+) state has three absorption bonds in the visible range. The longest wavelength bond is near 600nm in type a.Cyt, near 560nm in type.b and near 550nm in type.c.

The heme as prosthetic group to this cytochromes, but not covalently in Cyt.a and Cyt.b types. In Cyt.c case the heme group binds tightly by covalently through “Cys” residues. The Cytochromes of type a and b and some of type “C” are integral proteins of the inner mitochondrial membrane. Cyt.c of mitochondria, a soluble protein that associates through electrostatic interactions with the outer surface of the inner membrane.
(1) Cytochrome.a and a3:
- These are also called as “Cytochrome oxidase”. These are found solely in the mitochondria.
- It has molecular weight 72,000 (or) 93,000
- Oxidation potential of +0.29Volt.
- The reduced forms of cytochrome.a of animal tissue exhibit an α absorption band near 600nm.
- Cytochrome a and a3 possess and identical type of iron – porphyrin complex called “Heme.a”,but their location to apo-protein are different.
- One heme group is located along with one copper ion. This heme is called “heme.a”. This cyt.a functions as the “anaerobic oxidizing unit.
- The other heme.a called heme.a3 is located along with the second copper ion at the binding site for molecular O2 on subunit-I and functions as aerobic reducing unit of the enzyme complex.
- Cyt-a absorbs at 605,517 and 414nm where as Cyt.a3 absorbs at 600 and 445nm. Cyt .a doesn’t react with O2
- Cyt.oxidase thus constitute the last carrier in the chain of electron transport and is referred to as the “terminal oxidase4” of the cytochrome chain.
(2) Cytochrome-b:
- Cyt.b contains protoporphyrin IX complex, but the apoprotein is different.
- Cyt.b of animal tissue has α-absorption bands near 563nm, β-absorption bands near 530nm and γ-absorption near 430nm.
- It is thermo-stable and not easily extractable
- Oxidation potential is +0.04 volt in the mitochondria, and -0.34 volt when it is free.
- Cyt.b2,b3,b4 etc are found in microorganisms
- The Cyt.b doesn’t react with O2, CO (or) CN–
- Cyt+.b is reduced by accepting an electron from reduced “COQ”.
(3)Cytochrome.C:
- It is the best characterized of all cytochromnes.
- It is water soluble and easily extractable.
- The Cyt.C have α- absorption bands near 550nm, β-absorption bands near 521nm and γ-absorption near 416nm
- In Cyt.C heme is attached with protein by means of two thio-esther linkages involving sulfur of two cysteine and apoprotein.
- It is a basic protein with one polypeptide chain with 104a.as.
- Cyt.C acts as an electron carrier because its iron atom readily changes its valance from 3 to 2, i.e., Fe+3+ e––> e+2.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.