The electron carriers of the respiratory chain are organized into the membrane-embedded supramolecular complexes that can be physically separated.
Gentle treatment of the inner mitochondrial membrane with detergents allows the resolution of four unique electron – carrier complexes, each capable of catalyzing electron transfer through a portion of the chain.
Complex I and II catalyze electron transfer to ubiquinone from two different electron donors: NADH (complex.I) and succinate (Complex.II), Complex.III carriers electrons from ubiquinone to cytochrome.c, and complex. IV completes the sequence by transferring electrons from Cyt.C to O2
Read these notes before going to the mechanism
PROTEIN COMPONENTS OF THE MITOCHONDRIAL ETC
Electron Transport Chain Mechanism
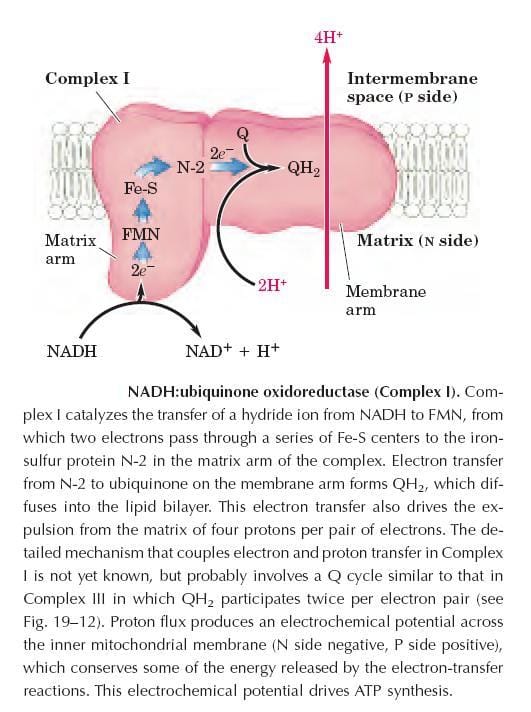
Complex I: NADH dehydrogenase
Complex-I also called “NADH: Ubiquinine oxidoreductase” is a large enzyme composed of 42 different polypeptide chains, including as FMN-containing flavoprotein and at least six iron-sulfur centers. The complex shows L-shaped, arm extending into the matrix.
Mechanism
Complex-I catalyzes the transfer of a hydride ion from NADH to FMN, from which two electrons pass through a series of Fe-S centers to the “iron-sulfur protein N-2 in the matrix arm of the complex.
Electron transfer from N-2 to ubiquinone on the membrane arm forms QH2, which diffuses into the lipid bilayer. It also drives the expulsion from the matrix of four protons per pair of electrons.
The detailed mechanism that couples electron and proton transfer in complex-I is not yet known, but probably involves a Q cycle similar to that in complex-III in which QH2 participates twice per electron pair.
This proton flux produces an electrochemical potential across the inner mitochondrial membrane (N-side negative, P-side positive), which conserves some of the energy released by the electron transfer reactions.
This electrochemical potential drives ATP synthesis. There is a large negative free energy change, the energy released is -12K.Cal/mol. Utilized by ADP&Pi forms ATP.
NADH -> FMN -> (Fe-S1) -> (Fe-S2) -> (Fe-S3) -> (Fe-S4) -> CoQ
Complex-II: Succinate dehydrogenase
Complex-II catalyzes the reduction of Co.Q by electrons remove from succinate.
Succinate + CoQ –> Fumarate + CoQ.H2
This complex which contains FAD is composed of four polypeptides with a molecular weight of 70,000, 27,000, 15,000 and 13,000. Succinate dehydrogenase, the only membrane-bound enzyme in the Citric acid cycle. Although smaller and simpler than complex-I, It contains two types of prosthetic groups and at least four different proteins.
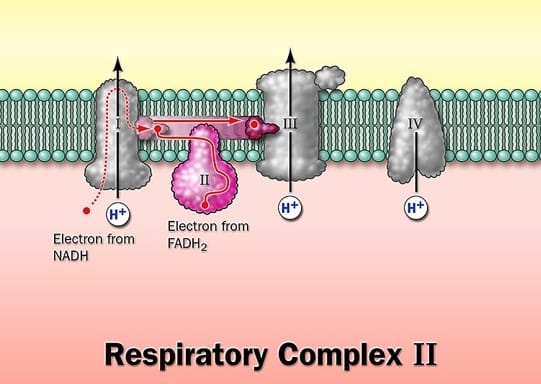
One protein has a covalently bound FAD and a Fe-S center with four Fe atoms; a second iron-sulfur protein is also present. Electrons pass from succinate to FAD, then through the Fe-S centers to ubiquinone.
Other substrates for mitochondrial dehydrogenases pass electrons into the respiratory chain at the level of ubiquinone, but not through complex-II.
The enzymes are “acyl~CoA dehydrogenase” and “Glycerol-3-Pdehydrogenase”.
The acyl~CoA dehydrogenase involving electron transfer proteins are “ETF (electron transferring flavoprotein): Ubiquinone oxidoreductase”. QH2 from all these reactions is re-oxidized by complex-III, the next component in the mitochondrial electron-transfer chain.
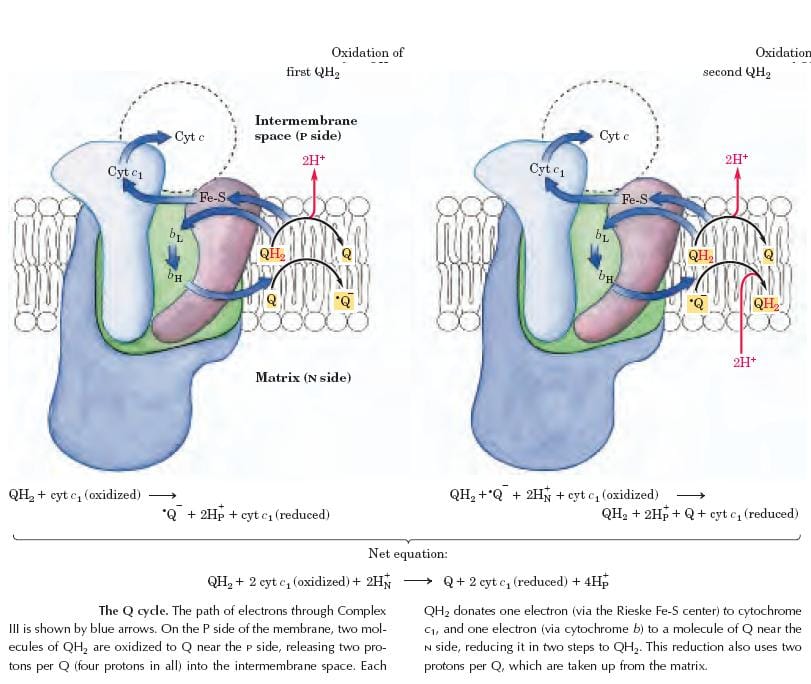
Complex-III: Ubiquinone: Cytochrome.C.Oxidoreductase
The Complex-III couples the transfer of electrons from ubiquinol(QH2) to cytochrome.C with the vectorial transport of protons from the matrix to the intermembrane space. This is a multi-protein complex, consisting of a cluster of iron-sulfur proteins, “Cyt.b” and “Cyt.C1”.
Cyt.b & C1 contain a heme prosthetic group. During this process of transfer of an electron, the iron in heme group shuttles between Fe+3 and Fe+2 forms.
The free energy change is -10Kcal/mol; one molecule of ATP is synthesized in this step.
Cytochrome.C
- It contains one heme, prosthetic group. The term cytochrome is derived from a greek word meaning “Cellular colors”. “Axel Theorell” isolated it.
- It is not a part of an enzyme complex, it moves between complex.III and IV as a freely soluble protein.
- Cyt.C collects electrons from the complex.III and delivers them to complex.IV.
- Cyt.C also the mediator of ‘apoptosis’ (Programmed cell death).
QH2 + Cyt.c -> Q + Cyt.C
(red) (oxi) (oxi) (red)
Complex-IV: Cytochrome Oxidase
In the final step of the respiratory chain, complex IV carries electrons from cytochrome.C to molecular oxygen, reducing it to H2O. The complex IV is tightly bound to the mitochondrial membrane.
Four electrons are accepted from Cytochrome.C, and passed on to molecular oxygen. Complex.IV also functions as a proton pump; free energy change is -24 Kcal/mol and 1ATP molecule is synthesized.
Cyt.oxidase contains two heme groups and two copper ions.
The two heme groups are structurally similar, but they are located at different parts of the enzyme complex and denoted as “Cyt.a” and “Cyt.a3”. The functional unit of the enzyme is a single protein and is referred to as Cytochrome-a,a3.
Path of electron through Complex-IV
The three proteins critical to electron flow are I, II and III. The lighter outline includes the other ten proteins in the complex. Electron transfer through complex-IV begins when two molecules of reduced Cyt.C each donates an electron to the binuclear center ‘CuA’. From here electrons pass through heme.a to the Fe-Cu center (Cyt.a3 & CuB).
Oxygen now binds to heme a3 and I reduced to its peroxy derivative (O22-) by two electrons from the Fe-Cu center. Delivery of two more electrons from Cyt.C converts the (O22-) to two molecules of water, consuming four “Substrate” protons from the matrix. At the same time, four more protons are pumped from the matrix by an as yet unknown mechanism.
Inhibitors of Electron Transport Chain
Transfer of electrons is selectively inhibited as various components of the electron transport chain by a variety of substances. Some of these are used as poisons (eg: insecticides) and some of which are used as drugs.
Site-I (Complex-I)
- Rotenone: A fish poison and also insecticide. Inhibits transfer of electrons through “Complex-I-NADH-Q-reductase”.
- Amobarbital (Amytal) and Secobarbital: Inhibits electrons transfer by competing with Co.Q.
- A: An antibiotic, Blocks electron transfer by competing with Co.Q.
- Drugs: Chlorpromazine and hypotensive drug-like guanethidine.
Step II (Complex-III)
- A
- BAL (dimo-Caprol)
- Hypoglycamic drugs: like phenformin
Step III (Complex-IV)
- Cyanide(CN–)
- H2S
- Azide (N3–)
- CO (Carbon monoxide): It inhibits Cyt .oxidase by combining with O2 binding site. It can be reversed by illumination with light.
Complex-II (Succinate dehydrogenase: ( FAD)
- Carboxin
- TTFA
- Malonate: A competitive inhibitor of succinate dehydrogenase
This special notes on Electron Transport chain mechanism and inhibitors are prepared for students. You can download this notes as Smartphone compatibility more by using the below Print Friendly icon.
Please share this useful notes with your friends through social media like Facebook, Twitter, and Pinterest. Just use the below Social Icons.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.