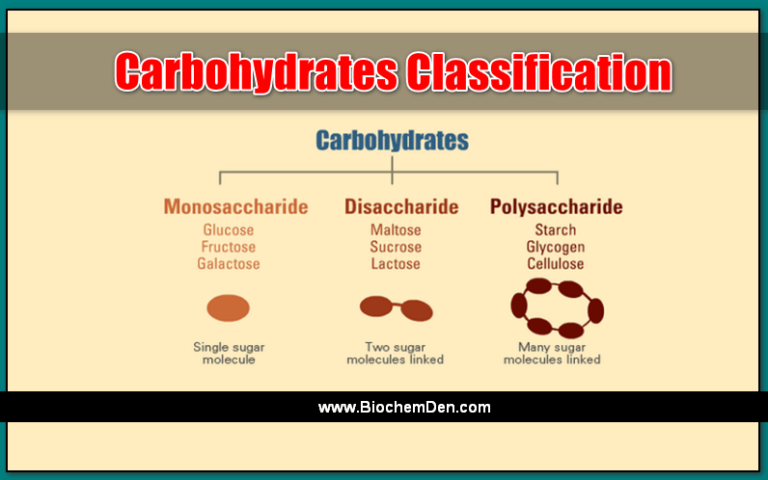
Carbohydrates composed of ten or more monosaccharide units are classified as polysaccharides, and their molecules are colloidal.
They may be considered condensation polymers in which the monosaccharides (or their derivatives, such as amino sugars and uronic acids) are joined by glycosidic linkages.

By far, the most important naturally occurring polysaccharides are starch and cellulose.
Another term for polysaccharides is “glycans”. According to Whistler’s classification, polysaccharides can be divided into two types. They are two types of polysaccharides
- HomoGlycans are made up of a single monosaccharide. Eg: starch, cellulose, glycogen, and Inulin.
- HeteroGlycans are composed of two or more kinds of monosaccharides (or their derivatives), such as mucopolysaccharides, and are called “heteroglycans.”
- The polysaccharides made with glucose are called “glucans.”
- The polysaccharides made with fructose are called “fructans.”
- The polysaccharides made with mannose are called “Mannans”.
- The polysaccharides made with Xylose are called “Xylans.”
What is the Classification of Polysaccharides?
When they are formed by the same monosaccharides, they are called homopolysaccharides, like starch, glycogen, and cellulose, each of which is formed by hundreds of molecules of glucose linked by glycosidic linkages.
If the polysaccharide molecules are formed by different monosaccharides, we consider them heteropolysaccharides. Hyaluronic acid, formed by thousands of alternative units of N-acetyl glucosamine and glucuronic acid, is an example of a heteropolysaccharide.
HOMOPOLYSACCHARIDES
a. Cellulose
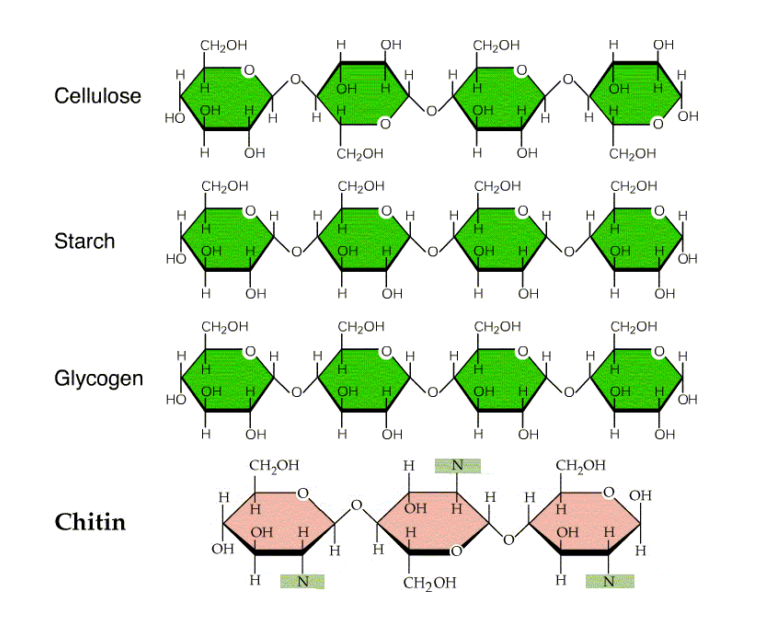
Cellulose is the main structural material of trees and other plants. The general formula of cellulose is (C6H10O5)n. Wood is 50% cellulose, while cotton wool is almost pure cellulose. Other sources of cellulose are straw, corncobs, bagasse, and similar agricultural wastes.
Structure of Cellulose
Cellulose is a linear polymer of D-glucose residues bonded by β(1, 4)-O-glycosidic linkages. It is the most abundant carbohydrate in nature.
It is formed by glucose units, linked by β-1, 4-O-glycosidic linkages.
We can say then, if we consider the linkage, that the repeating unit in cellulose is cellobiose, the disaccharide formed by two molecules of glucose linked by Beta-D-O glycosidic bonds, (that is why some textbooks say that the monomer in cellulose is cellobiose).
The long fibers of cellulose are held together by intermolecular hydrogen bonds.
Hydrogen bonding continues in the same plane with other chains and in planes above and below this plane to form strong, fibrous bundles. It made cellulose very appropriate for its structural function in plants.
Since cellulose is formed by glucose molecules, it can be a source of energy for certain species.
The lack of human beings’ enzymes for digesting cellulose makes this polysaccharide unsuitable for human nutrition (Have you thought about how hunger in the world could disappear if we had enzymes for digesting cellulose?).
Properties of Cellulose
Cellulose is a colorless amorphous solid having no melting point. It decomposes under strong heating. It is insoluble in water and most organic solvents. However, it dissolves in Schweitzer’s reagent, which is an ammoniacal solution of cupric hydroxide.
B. Starch
Starch is the second most abundant carbohydrate in nature. It is the main contributor to carbohydrates in our diet.
It exists only in plants, and it is stored in the seeds, roots, and fibers as a food reserve. The chief sources of starch are cereals, potatoes, corn, and rice.
The biological functions include, in plants, the main means of storage of sugar, and of energetic sources; in humans, the first supply of glucose during dieting.
Structure of Starch
Amylose starch is actually a mixture of two structurally different polysaccharides: Amylose (20%) and Amylopectin (80%).
When starch is heated with hot water, it can be separated into these components. The part soluble in water is amylose, and the remaining fraction is amylopectin.
Both amylose and Amylopectin are composed of D-Glucose units.
Amylose
- Amylose is a linear molecule formed by D-glucose units linked by α-glycosidic linkages.
- It is made up of D-Glucose units joined by α-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit.
- The number of D-Glucose units in Amylose ranges from 60 to 300
- The structure of the amylose molecule is helicoidal.
Amylopectin
- Amylopectin is the second molecule that forms starch.
- It has a branched-chain structure.
- They compose it of chains of 25 to 30 D-Glucose units joined by α-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit.
- These chains are connected to each other by 1,6-linkages.
- The number of D-glucose units in Amylopectin ranges from 300 to 6000.
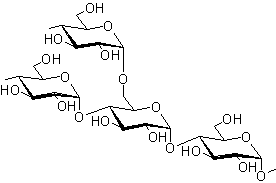
The disaccharides that can be obtained from the digestion of amylopectin are maltose and isomaltose.
C. Glycogen
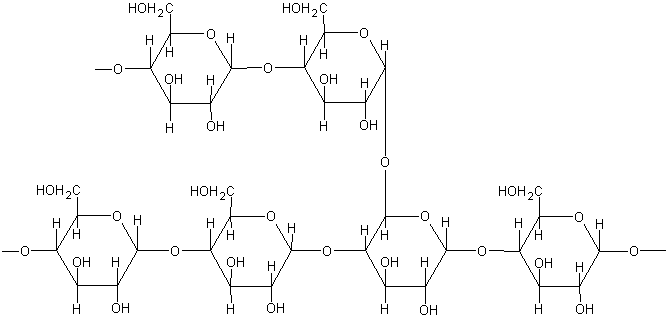
- Glycogen is a food storage polysaccharide.
- It is called “animal starch.”
- Glycogen is first found in the liver and muscles. It stores energy in mammals.
- It occurs in animal cells as particles much smaller than starch grains.
- The structure of glycogen is like that of Amylopectin.
- Each branch chain contains 10 to 20 glucose units.
- Its molecular weight is 1-2 X 107
- According to physical properties, it is a white powder and more soluble in water than amylopectin.
- When hydrolyzing the glycogen, it yields maltose units.
The structure of glycogen is very similar to amylopectin but more branched, with one branch every 8 to 12 glucose units.
Glycogen is the way in which glucose is stored in animals. Glycogen is stored mainly in the liver (to release glucose into the blood when necessary) and in muscles, where it is used as a reserve of energy for muscular contraction.
HETERO POLYSACCHARIDES
Heteropolysaccharides contain two or more different monosaccharides. Usually, they provide extracellular support for organisms of all kingdoms: the bacterial cell envelope, or the matrix that holds individual cells together in animal tissues, and provides protection, shape, and support to cells, tissues, and organs.
Heteropolysaccharides provide extracellular support to very different organisms, from bacteria to humans; together with fibrous proteins, like collagen, elastin, fibronectin, laminin, and others, heteropolysaccharides are the most important components of the extracellular matrix.
Hyaluronic acid, chondroitin sulfates, and dermatan sulfates are important heteropolysaccharides in the extracellular matrix.
These heteropolysaccharides are usually formed by the repetition of a disaccharide unit of amino sugar and acid sugar.
Other common constituents are sulfate groups linked to certain monosaccharides.
Usually, heteropolysaccharides are associated with proteins, forming proteoglycans, glycosaminoglycans or mucopolysaccharides (since they are abundant in mucous secretions).
As a group, they perform diverse functions: structural, water metabolism regulation (as a reservoir of water), cellular cement, biological sieve, biological lubricant, docking sites for growth factors, among other functions.
Established specific functions of some glycosaminoglycans are:
a. Hyaluronic Acid (Hyaluronate)
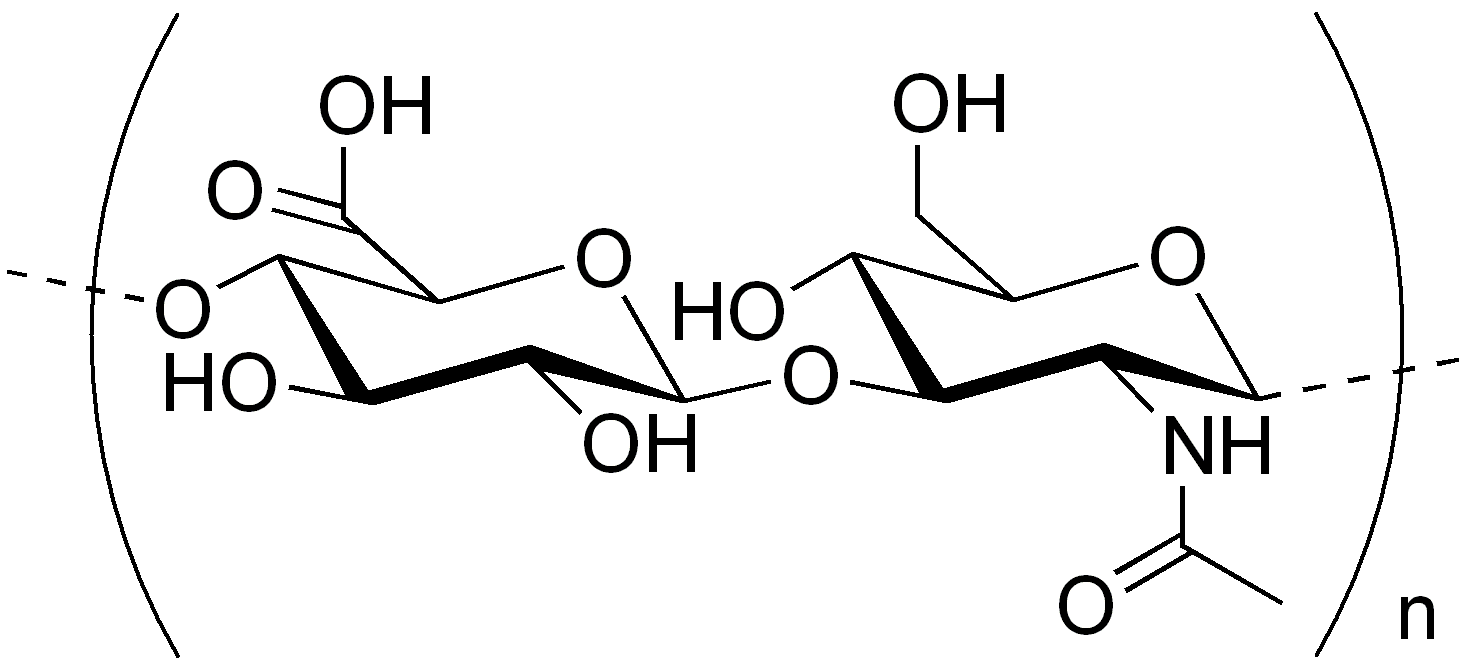
- Hyaluronic acid is the most abundant member of mucopolysaccharides and is found in higher animals as a component of various tissues, such as the vitreous body of the eye, the umbilical cord, and the synovial fluid of joints.
- The synovial fluid is viscous in nature and functions as a biological lubricant. The viscosity nature is because of the presence of a high amount of hyaluronic acid (about 0.03%).
- It is a straight-chain polymer of D-Glucuronic acid and N-acetyl-D-glucosamine (NAG) alternating in the chain.
- The molecular weight is approximately 5,000,000.
- The chain is formed by β-1→3 and β-1→4.
- It is an acidic substance because the carboxyl groups are largely ionized at the cellular pH.
- It is a lubricant in the synovial fluid of joints.
- Furthermore, it gives consistency to the vitreous humor and contributes to the tensile strength and elasticity of cartilage and tendons.
b. Chondroitin Sulfates
The chondroitin sulfates are among the principal mucopolysaccharides in the ground substance of mammalian tissues and cartilage and occur combined with proteins.
Three different chondroitin sulfates have been isolated and designated as A, B, and C.
- Chondroitin sulfate A: This is the chief one present in cartilage, adult bone, and the cornea.
- Chondroitin sulfate B: It is present in skin, heart valves, and tendons.
- Chondroitin sulfate C: It is found in cartilage and tendons.
A, B, and C have been differentiated because of optical rotation and their behavior towards the testicular hyaluronidase enzyme.
Chemistry:
- Chondroitin sulfate A and C appear to comprise equimolar quantities of N-acetyl-2-amino-2-deoxy-D-Galactose (N-acetyl-D-galactosamine or N-acetyl-chondrosamine), D-glucuronic acid, and sulfuric acid.
- A deacylated disaccharide, called “Chondrosin”, has been obtained by the acid hydrolysis of Chondroitin sulfate A.
- Chondrosin is 3-O-β-D-glucopyranosyluronic acid-2-amino-2-deoxyD-galactopyranose.
- The structure of chondroitin sulfate C is the same as that of chondroitin sulfate A, except that the sulfate group is at position 6 of the galactosamine group instead of at position 4.
- Highly purified bovine cartilage chondroitin sulfate was found to have a molecular weight of 43,300 by osmotic measurements.
- Chondroitin sulfate B is also known as β-heparin, and, more recently, dermatan sulfate (from the skin), is the sulfate of a polysaccharide composed of L-iduronic acid and N-Acetylgalactosamine.
c. Dermatan sulfate
- It (former chondroitin sulfate B) is found mainly in the skin, but also in vessels, the heart, and the lungs. It may be related to coagulation, vascular diseases, and other conditions.
- Furthermore, it is a mucopolysaccharide structurally similar to Chondroitin sulfate A except that the D-Glucuronic acid is replaced by L-iduronic acid (the two uronic acids differ in configuration only at C5).
- It involves the two linkages in α-1→3 and β-1→4. Dermatan sulfate is also known by its conventional name, Condroitin Sulfate B.
- It is a misnomer, since it differs from both Chondroitin Sulfate A and C in the composition of their repeating disaccharide units.
d. Keratan sulfate
- Kerato sulfate differs from other mucopolysaccharides in that the uronic acid component is replaced by D-galactose.
- The second acetylated amino sugar component (which is N-acetyl-D-glucosamine in this case) is esterified by a sulfate group at carbon 6. However, the two alternating linkages involved are β-1→4 and β-1→3.
- Here, the linkage between the repeating disaccharide units are β-1→3 rather than β-1→4
- present in the cornea, cartilage bone, and a variety of other structures such as nails and hair.
e. Heparin
- Heparin is a blood anticoagulant present in the liver (from which it was originally isolated), lung, thymus, spleen, and blood.
- It is a polymer of D-glucuronic acid and D-glucosamine.
- The molecular weight of heparin is 17,000 to 20,000.
- It is strongly acidic because of its sulfuric acid groups and readily forms salts. The barium salt is used in its isolation.
- It is a potent natural anticoagulant produced in the mast cells that causes antithrombin bind to thrombin and inhibit blood coagulation.
Final words about Polysaccharides
Glycosaminoglycans are made in the endoplasmic reticulum (ER) and the Golgi. Lysosomal hydrolases break them down. Mucopolysaccharidosis is caused by a lack of one hydrolase enzyme.
Glycosaminoglycans accumulate in tissues in these hereditary disorders, resulting in skeletal and extracellular matrix deformities, as well as mental retardation.
Examples of these genetic diseases are Hunter and Hurler syndromes.
Physical deformities, mental retardation, and disturbances in the degradation of heparan sulfate and dermatan sulfate are all symptoms of these diseases, which are caused by different enzyme deficiencies.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.