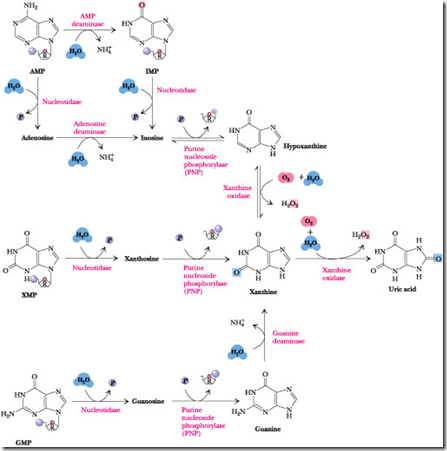
Purine catabolism pathway is one of the Nucleic acid Metabolism. Because nucleic acids are ubiquitous in cellular material, significant amounts are ingested in the diet. Nucleic acids are degraded in the digestive tract to nucleotides by various nucleases and phosphodiesterases. Nucleotides are then converted to nucleosides by base-specific nucleotidases and nonspecific phosphatases.
Nucleosides are hydrolyzed by Nucleosidases or Nucleoside phosphorylases to release the purine base.
NMP + H2O → Nucleoside + Pi
The pentoses liberated in these reactions provide the only source of metabolic energy available from purine nucleotide degradation. Feeding experiments using radioactively labeled nucleic acids as metabolic tracers have demonstrated that little of the nucleotide ingested in the diet is incorporated into cellular nucleic acids.
- Nucleic Acids: The Molecular Life Language Basics in Biology
- Basic Components of Nucleic Acids – Purines and Pyrimidines
These findings confirm the de novo pathways of nucleotide biosynthesis as the primary source of nucleic acid precursors. Ingested bases are, for the most part, excreted. Nevertheless, cellular nucleic acids do undergo degradation in the course of the continuous recycling of cellular constituents.
The Major Pathways of Purine Catabolism Leads to Uric Acid
The various nucleotides are first converted to nucleosides by intracellular nucleotidases. These nucleotidases are under strict metabolic regulation so that their substrates, which act as intermediates in many vital processes, are not depleted below critical levels.
- Purine Synthesis: Synthesis of Purine RiboNucleotides
- Pyrimidine Synthesis Pathway: Synthesis of pyrimidine molecules
Nucleosides are then degraded by the enzyme Purine Nucleoside Phosphorylase (PNP) to release the purine base and Ribose-l-P. Note that neither adenosine nor deoxyadenosine is a substrate for PNP. Instead, these nucleosides are first converted to inosine by adenosine deaminase. The PNP products are merged into xanthine by guanine deaminase and xanthine oxidase, and xanthine is then oxidized to uric acid by this latter enzyme.
Steps involved in Purine Catabolism
The major pathways of Purine catabolism pathway and deoxynucleotide catabolism in animals are explained in 3 stages.
- Dephosphorylation
- Deamination
- Glycosidic bond cleavages
Steps involved in Purine catabolism:
Step 1: Dephosphorylation:
The purine molecules AMP, IMP, XMP, and GMP are dephosphorylated into their corresponding nucleotides such as Adenosine, Inosine, Xanthosine, Guanosine. This reaction is catalyzed by the enzyme “Nucloetidase”
Step 2: Deamination:
The AMP (Nucleotide) and Adenosine (Nucleoside) is deaminated into IMP and Inosine. This reaction is catalyzed by AMP deaminase and Adenosine deaminase. Guanine (nitrogenous base only) is deaminated into Xanthosine in the presence of the “Guanosine deaminase”.
Step 3: Glycosidic bond cleavage:
The nucleoside Inosine, Xanthosine, Guanosine is converted into Hypoxanthine, Xanthine, and Guanine. This reaction is catalyzed by “Purine nucleotide phosphorylase”.
- In this step, the Glycosidic linkage which is present both N9 of Nitrogenous base and C1 of Sugar molecule will be breath.
- The hypoxanthine is converted into Xanthine. The Xanthine is converted into Uric acid. This reaction is catalyzed by Xanthine Oxidase (Which is mini electron transport system).
Fate of Uric Acid:
 Severe Combined Immuno Deficiency (SCID) Syndrome:
Severe Combined Immuno Deficiency (SCID) Syndrome:
A Lack of Adenosine Deaminase Is One Cause of This Inherited Disease Severe combined immunodeficiency syndrome, or SCID is a group of related inherited disorders characterized by the lack of an immune response to infectious disease.
This immunological insufficiency is attributable to the inability of B and T lymphocytes to proliferate and produce antibodies in reaction to an antigenic challenge. About 30% of SCID patients suffer from a deficiency in the enzyme adenosine deaminase (ADA).

ADA deficiency is also implicated in a variety of other diseases, including AIDS, anemia, and various lymphomas and leukemias. Gene therapy, the repair of a genetic deficiency by the introduction of a functional recombinant version of the gene, has been attempted on individuals with SCID due to a defective ADA gene. ADA is a Zn2+-dependent enzyme, and Zn2+ deficiency can also lead to reduced immune function.
The effect of elevated levels of deoxyadenosine on purine metabolism. If ADA is deficient or absent, deoxyadenosine is not converted into deoxyinosine as normal. Instead, it is salvaged by a nucleoside kinase, which converts it to dAMP, leading to accumulation of dATP and inhibition of deoxynucleotide synthesis. Thus, DNA replication is stalled.
- 10 Google Best Chrome Extensions for Biology Students
- Amino acids Lecture Chart from Biochemden Gallery
In the absence of ADA, deoxyAdenosine is not degraded but instead is converted into dAMP and then into dATP. dATP is a potent feedback inhibitor of deoxyNucleotide biosynthesis (discussed later in this chapter). Without deoxyRibonucleotides, DNA cannot be replicated and cells cannot divide. Rapidly proliferating cell types such as lymphocytes are particularly susceptible if DNA synthesis is impaired.
Xanthine Oxidase:
Xanthine oxidase is present in large amounts in liver, intestinal mucosa, and milk. It oxidizes hypoxanthine to xanthine and xanthine to uric acid. Xanthine oxidase is a rather indiscriminate enzyme, using molecular oxygen to oxidize a wide variety of purines, pteridines, and aldehydes, producing H2O2 as a product. Xanthine oxidase possesses FAD, non-heme Fe-S centers, and a molybdenum cofactor ) as electron-transferring prosthetic groups.
In humans and other primates, uric acid is the end product of purine catabolism and is excreted in the urine. Birds, terrestrial reptiles, and many insects also excrete uric acid, but, in these organisms, uric acid represents the major nitrogen excretory compound, because, unlike mammals, they do not also produce urea.
Instead, the catabolism of all nitrogenous compounds, including amino acids, is channeled into uric acid. This route of nitrogen catabolism allows these animals to conserve water by excreting crystals of uric acid in paste-like solid form.
Gout: An Excess of Uric Acid
 Gout is the clinical term describing the physiological consequences accompanying excessive uric acid accumulation in body fluids. Uric acid and urate salts are rather insoluble in water and tend to precipitate from solution if produced in excess. The most common symptom of gout is arthritic pain in the joints as a result of urate deposition in cartilaginous tissue. The joint of the big toe is particularly susceptible.
Gout is the clinical term describing the physiological consequences accompanying excessive uric acid accumulation in body fluids. Uric acid and urate salts are rather insoluble in water and tend to precipitate from solution if produced in excess. The most common symptom of gout is arthritic pain in the joints as a result of urate deposition in cartilaginous tissue. The joint of the big toe is particularly susceptible.
Urate crystals may also appear as kidney stones and lead to painful obstruction of the urinary tract. Hyperuricemia, chronic elevation of blood uric acid levels, occurs in about 3% of the population as a consequence of impaired excretion of uric acid or overproduction of purines. Purine-rich foods (such as caviar—fish eggs rich in nucleic acids) may exacerbate the condition. The biochemical causes of gout are varied. However, a common treatment is allopurinol. This hypoxanthine analog binds tightly to xanthine oxidase, thereby inhibiting its activity and preventing uric acid formation. Hypoxanthine and xanthine do not accumulate to harmful concentrations because they are more soluble and thus more easily excreted.
The Fate of Uric Acid:
The subsequent metabolism of uric acid in organisms. In mollusks and in mammals other than primates, uric acid is oxidized by urate oxidase to allantoin and excreted. In bony fishes (teleosts), uric acid degradation proceeds through yet another step wherein allantoin is hydrolyzed to allantoic acid by allantoinase before excretion. Cartilaginous fish (sharks and rays), as well as amphibians, further degrade allantoic acid via the enzyme, allantoicase, to liberate glyoxylic acid and two equivalents of urea.
In humans and other primates, the final product of purine catabolism is Uric acid, which is excreted in the Urine. The uric acid appears to play a role beyond that of an end product of purine metabolism.
Uric acid is excreted end product if urine catabolism in primates, birds and some other animals, but in many other vertebrates it is further degraded to Allantoin by the action of Urate Oxidase. In other organisms, the pathway is further extended. A normal adult human excretes Uric acid at a rate of about 0.6g/24 h; the excreted product arises in part from ingested purines and in part from a turnover of the Purine nucleotides of nucleic acids.
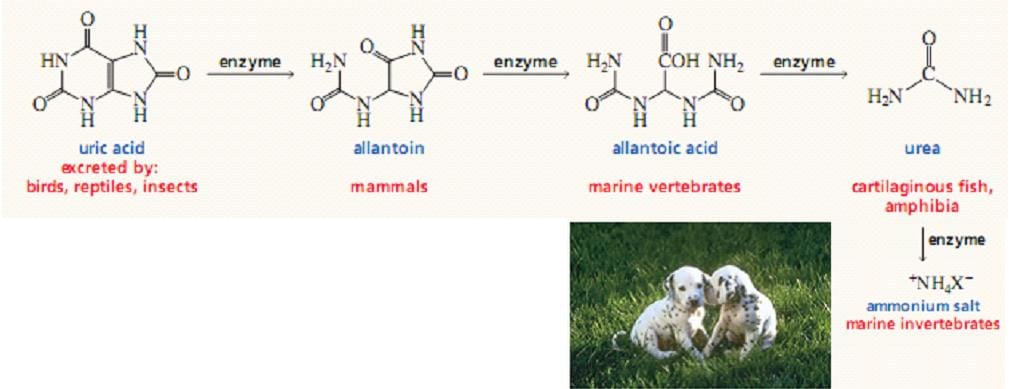
Even simpler animals, such as most marine invertebrates (crustacea and so forth), use urease to hydrolyze urea to CO2 and ammonia. In contrast to animals that must rid themselves of potentially harmful nitrogen waste products, microorganisms often are limited in growth by nitrogen availability.
Many possess an identical pathway of uric acid degradation, using it instead to liberate NH3 from uric acid so that it can be assimilated into organic-N compounds essential to their survival.
Additional Points:
- Genetic abbreviations in human purine metabolism have been found, some with serious consequences. For example, adenosine deaminase deficiency leads to severe immunodeficiency disease in which T-lymphocytes and B-Lymphocytes do not develop properly.
- Lack of adenosine deaminase leads to a 100-fold increase in the cellular concentration of dATP, a strong inhibitor of Ribonucleotide reductase.
- High levels of dATP produce a general deficiency of other dNTPs in T-lymphocytes.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.