What are the essential components of Spectrophotometer instrumentation? What is Electromagnetic radiation? Electromagnetic radiation has been put to many uses in our daily routine.
Radio and television broadcasting, medical x-ray, etc. are some common examples.

Electromagnetic radiation in analytical chemistry gained much importance during the last 50 years to characterize materials.
Electromagnetic radiation in the region of 200 to 700nm is generally termed light.
The eye can perceive radiation between 340 to 650nm and can distinguish it as various (VIBGYOR).
What is A Spectrophotometer?
A spectrophotometer can be located in many studies, biology, chemistry, and industrial laboratories. The spectrophotometer is utilized for research and data evaluation in different scientific fields.
Some of the significant fields in which a spectrophotometer is employed are physics, molecular biology, chemistry, and biochemistry labs. Generally, the title refers to Ultraviolet-Visible (UV-Vis) Spectroscopy.
What a spectrophotometer does is transmit and receive light. The spectrophotometer is utilized to evaluate test material samples by passing light using the sample and studying the intensity of the wavelengths.
Different samples modify the light in numerous distinct ways, and this allows researchers to obtain much more facts about the check content by viewing the change in light conduct as it passes by way of the sample.
These final results must be precise, or the researcher will just be throwing away time making use of a flawed instrument. The only way to make sure accuracy is by executing a spectrophotometer calibration.
What is VIBGYOR (or) ROYGBIV?
ROYGBIV or Roy G. Biv is an acronym for the sequence of hues commonly described as making up a rainbow: Red, Orange, Yellow, Green, Blue, Indigo, and Violet.
A rainbow spans a continuous spectrum of colours; the distinct bands are artefacts of human colour vision. In ROYGBIV, the colours are arranged to decrease wavelengths, with red being 650 nm and violet being about 400 nm.
A Spectrophotometer has all the basic components of a photoelectric colorimeter with more sophistication.
The instruments that are used to study the absorption (or) emission of electromagnetic radiation as a function of wavelength are called “SPECTROMETERS” or “SPECTROPHOTOMETERS”.
History of Spectrophotometers
For millions of years, light has defined the life of Homo sapiens. Through photosynthesis, light has given us food, energy, and the atmosphere.
Moreover, we communicate information using light, see the big objects far from us through the telescope and small objects through the microscope.
From where does light get this transcending power?
It took nearly a millennium until James Clark Maxwell, in 1864, told the world that light is made of waves of disturbances of electric and magnetic fields.

Principle
What is the Principle of a Spectrophotometer? The Spectrophotometer is a much more refined version of a colourimeter. In a colorimeter, filters are used, which allow a broad range of wavelengths to pass through. In contrast, in the Spectrophotometer, a prism (or) grating is used to split the incident beam into different wavelengths. By suitable mechanisms, waves of specific wavelengths can be manipulated to fall on the test solution. The range of the wavelengths of the incident light can be as low as 1 to 2nm. The Spectrophotometer helps measure the absorption spectrum, the absorption of light by a solution at each wavelength. It is the basic Principle of spectrophotometry in biochemistry.
Spectrophotometer Instrumentation
The essential components of spectrophotometer instrumentation include:
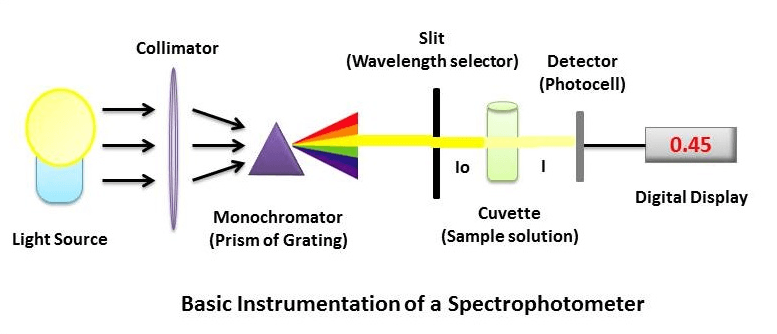
- A Stable and cheap radiant energy source
- A monochromator to break the polychromatic radiation into component wavelength (or) bands of wavelengths.
- Transport vessels (cuvettes) to hold the sample
- A Photosensitive detector and an associated readout system
1. Radiant Energy Sources
Materials that can be excited to high energy states by a high-voltage electric discharge (or) by electrical heating serve as excellent radiant energy sources.
- Sources of Ultraviolet radiation: The most commonly used sources of UV radiation are the hydrogen lamp and the deuterium lamp. Xenon lamp may also be used for UV radiation, but the radiation produced is not as stable as the hydrogen lamp.
- Sources of Visible radiation: “Tungsten filament” lamp is the most commonly used source for visible radiation. It is inexpensive and emails continuous radiation in the range between 350 and 2500nm. “Carbon arc”, which provides more intense visible radiation, is used in a few commercially available instruments.
- Sources of IR radiation: “Nernst Glower” and “Global” are the most satisfactory sources of IR radiation. Global is more stable than the nearest flower.
2. Wavelength selectors
Wavelength selectors are of two types.
- Filters: “Gelatin” filters are made of a gelatin layer, coloured with organic dyes and sealed between glass plates.
- Monochromators: A monochromator resolves polychromatic radiation into its wavelengths. It isolates these wavelengths into very narrow bands. The essential components of a monochromator are.
- The entrance slip-admits polychromatic light from the source.
- Collimating device–Collimates the polychromatic light onto the dispersion device.
- Wavelength resolving device like a PRISM (or) a GRATING
- A focusing lens (or) a mirror
- An exit slip–allows the monochromatic beam to escape.
The kinds of resolving element are of primary importance
- PRISMS
- GRATINGS
PRISMS:
A prism disperses polychromatic light from the source into its constituent wavelengths under its ability to reflect different wavelengths to a different extent;
The degree of dispersion by the Prism depends on upon
- The optical angle of the Prism (usually 600)
- The material of which it is made
Two types of Prisms are usually employed in commercial instruments—namely, 600 cornu quartz prism and 300 Littrow Prism.
GRATINGS:
Gratings are often used in the monochromators of spectrophotometers operating ultraviolet, visible and infrared regions.
3. Sample Containers
Sample containers are also one of the parts of Spectrophotometer instrumentation. Samples to be studied in the ultraviolet (or) visible region are usually glasses (or) solutions and are put in cells known as “CUVETTES”.
Cuvettes meant for the visible region are made up of either ordinary glass (or) sometimes Quartz. Most of the spectrophotometric studies are made in solutions. The solvents assume prime importance.
The essential factor in choosing the solvent is that the solvent should not absorb (optically transparent) in the same region as the solute.
4. Detection Devices
Most detectors depend on the photoelectric effect. The current is then proportional to the light intensity and, therefore, a measure of it. Essential requirements for a detector, including
- High sensitivity to allow the detection of low levels of radiant energy
- Short response time
- Long-term stability
- An electric signal which easily amplified for a typical readout apparatus.
5. Amplification and Readout
Radiation detectors generate electronic signals which are proportional to the transmitted light. These signals need to be translated into a form easy to interpret. It is accomplished by using amplifiers, Ammeters, Potentiometers and Potentiometric recorders.
The above five significant parts are a significant part of Spectrophotometer instrumentation. Now let us see the Applications of the Spectrophotometer.
What Is Spectrophotometer Calibration?
Spectrophotometer calibration is a procedure in which a researcher or scientist utilizes a calibration standard to check the accuracy of the light source. This process is essential to make sure that the spectrophotometer is operating correctly and the measurements are correct. The calibration method varies somewhat for various instruments. Most important, producers present a comprehensive calibration guide in the owner’s manual so that researchers know how to calibrate the equipment properly.
We are maintaining a calibration log that is also critical to display when and who performed the last calibration.
Resources Utilized to Calibrate Spectrophotometer
Spectrophotometer calibration filters, a.k.a neutral density filters, are mainly used to calibrate various transmittance values and are derived from NIST (National Institute of Standards and Technology). Some of NIST’s requirements consist of SRM 2031, 2034, NIST930e, etc.
Some spectrophotometer suppliers recommend that researchers send the machine in to be calibrated. The difficulty with sending the machine is the cost o research time, shipping expenditures, and other outside influences. It is ideal and most practical to calibrate a spectrophotometer without sending it out of the lab.
Utilizing Neutral Density Filters To Calibrate Spectrophotometer
For decades liquid calibration standards have been utilized. Starting in 2010, solid-state filters commenced replacing the liquid filters due to their potential never to be re-calibrated or replaced. Solid neutral density filters are also effortless to manage and will not break if they are accidentally dropped or mishandled.
The solid-state spectrophotometer calibration’s neutral density filters can be checked for photometric accuracy as well as stray light. To make sure a hundred accuracy, testing is performed at a minimum of 5 checkpoints. These spectrophotometer calibration filters can be used to calibrate devices made by Thermo Scientific, Beckman Coulter, Hitachi, Perkin Elmer, Hewlett Packard, Agilent, Shimadzu and far more.
How to Calibrate Making use of Neutral Density Filters?
In spectrophotometer calibration, a reference is used to zero out the instrument. When utilizing neutral density filters, no particular filters are required to zero out the instrument. To calibrate the machine, place the neutral density filter within the spectrophotometer, zero out the settings, and run the instrument. The final results need to be compared to a calibration certificate provided by the manufacturer of the calibration standards. If the final results are inside the tolerance array specified by the manufacturer, then the spectrophotometer is calibrated correctly.
Before calibrating a spectrophotometer and doing specific correct readings, it should be permitted to warm up before use. Most models take about 10 minutes or so to warm up. Spectrophotometer calibration must not be completed whilst the devices are warming up. If somebody tries to calibrate the spectrophotometer in the warm-up phase, it will throw the settings off.
SpectroPhotometer Applications
How to use the spectrophotometer? There are uses of spectrophotometry in biochemistry which are listed below:
1. Qualitative Analysis
The visible and UV spectrophotometer may be used to identify classes of compounds in both the pure and biological preparations. This is done by plotting absorption spectrum curves. Absorption by a compound in different regions gives some hints to its structure.
| Absorption Range (nm) | Structure (or) Type of compounds |
| 220 to 280nm | Aliphatic (or) alicyclic hydrocarbons (or) their derivatives |
| 220 to 250 nm | The compounds contain two unsaturated linkages in conjugation. Also, be due to “Benzene derivatives.” |
| 250 to 330 nm | The presence of more than two conjugated double bonds usually gives rise to absorption. |
| 450 to 500nm | Beta-carotene, a precursor of Vitamin A has eleven double bonds in a conjugated system and appears yellow. |
| 250 to 330 nm (249nm; 260nm and 325nm) | Vitamin K1 (Due to the presence of “NAPTHAQUINONE”) |
2. Quantitative Analysis
Spectrophotometer uses in the Quantitative analysis of Biochemistry practicals. Quantitative analysis method developing for determining an unknown concentration of a species by absorption spectrometry.
Most of the organic compounds of biological interest absorb in the UV-visible range of the spectrum.
Thus, several important classes of biological compounds may be measured semi-quantitatively using the UV-visible spectrophotometer. Nucleic acids at 254nm protein at 280nm provide good examples of such use.
The absorbance at 280nm by proteins depends on their “Tyrosine” and “Tryptophan” content.
- Estimation of Proteins by Lowry method
- Estimation of Tyrosine by Folin-Ciocalteau Method
- Estimation of Blood Glucose level by Folin-Wu method
3. Enzyme Assay
It is the primary application of spectrophotometry. This assay is carried out most quickly and conveniently when the substrate (or) the product is colour (or) absorbs light in the UV range.
Eg 1: Lactate Dehydrogenase (LDH)
Lactate + NAD + ↔ Pyruvate + NADH + H+
- The LDH is engaged in the transfer of electrons from lactate to NAD+.
- The products of the reaction are pyruvate, NAD, and a proton
- One of the products, NADH, absorbs radiation in the UV range at 340 nm while its oxidized counterpart, NAD+, does not.
- The forward reaction can be followed by measuring the increment in the light absorption of the system at 540nm in a spectrophotometer.
Eg 2: Pyruvate Kinase
Phosphoenolpyruvate + ADP ↔ Pyruvate + ATP
Pyruvate + NADH + H+ ↔ Lactate + NAD +
We have added a significant excess of NADH to the system. The system now absorbs at 340nm. According to the above-given reactions, each molecule of Pyruvate formed in the reaction. A molecule of NADH is oxidized to NAD+ in the second reaction when the system converts Pyruvate to locate.
Since NAD+ does not absorb at 340nm, the absorbance goes on decreasing with increased pyruvate generation. Such measurements are known as “Coupled assays”.
Sample enzymatic assays:
- Assay of Urease Enzyme Activity
- Assay of Salivary Amylase enzyme activity
- Effect of Temperature on Amylase activity
4. Molecular Weight determination
Molecular weights of amine picrates, sugars and much aldehyde and ketone compounds have been determined by this method. Molecular weights of only small molecules may be determined by this method.
- Study of Cis-Trans Isomerism: Geometrical isomers differ in the spatial arrangement of groups about a plane. The absorption spectra of the isomers also differ. The trans-isomer is usually more elongated than its cis counterpart. Absorption spectrometry can be utilized to study Cis-Trans isomerism.
- Control of Purification: Impurities in a compound can be detected very easily by spectrophotometric studies. “Carbon disulfide” impurity in carbon tetrachloride can be detected easily by measuring absorbance at 318nm, where carbon sulfide absorbs. A lot many commercial solutions are routinely tested for purity spectroscopically.
5. Other Physiochemical Studies
Spectrophotometry (UV-VIS) has been used to study the following physiochemical phenomena:
- Heats of formation of molecular addition compound and complexes in solution
- Determination of the empirical formula
- Formation constants of complexes in solution
- Hydration equilibrium of carbonyl compounds
- Association constants of weak acids and bases in organic solvents
- Protein-dye interactions
- Chlorophyll-Protein complexes
- Vitamin-A aldehyde–Protein complex
- Determination of reaction rates
- Dissociation constants of acids and bases
- Association of cyanine dyes
These are the basic spectrophotometer instrumentation and its applications.
Reference: Spectrophotometry
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.