Myoglobin (Mb) is a globular protein in skeletal muscle that plays an essential role in aerobic metabolism or energy production. The function of Mb has long remained largely unknown until recently, and it is one of the muscle tissue proteins.
Researchers at Harvard Medical School discovered that Mb carries oxygen from muscle cells through capillaries to the body’s other organs. In addition, they found out that Mb also transports carbon dioxide back into muscle cells during periods of exercise. They named the new protein myoglobin.
After years of research, scientists finally figured out the name of this protein, and now we know why it exists. This discovery gives us insight into the reasons behind the physiological changes that occur after strenuous exercise.
Moreover, researchers believe that these findings might lead to some breakthroughs in treating heart diseases and other metabolic disorders.
Myoglobin is a protein produced by myofibrils. Myofibrils are the proteins that form the structural core of every muscle fiber. Myofibrils contain sarcomeres, tiny repeating units of contractile structures. Each sarcomere consists of two main proteins: actin and myosin.
- Actin is a globular protein located within the inner part of each sarcomere and provides the backbone for the entire sarcomere structure.
- Myosins are rod-shaped proteins that constitute the thick filaments attached to actin filaments. These thin filaments extend between adjacent sarcomeres.
What is MyoGlobin?
Myoglobin is a globular protein found in muscle tissue that helps to store and transport oxygen. It is similar to hemoglobin, found in red blood cells, but myoglobin is much more abundant in muscle tissue. Myoglobin is responsible for the red color of muscle tissue and is essential for muscle function. When muscle cells do not have enough oxygen, they cannot function properly and eventually die. Myoglobin plays a vital role in ensuring that muscle cells have the oxygen to work correctly.
One of the essential proteins in the human body is myoglobin (symbol as MB or Mb). Today we are discussing myoglobin in this post.
It is found in muscle tissue, where it binds oxygen, helping to provide extra oxygen to release energy to muscular power contractions.
History of Myoglobin
Let us come to the history of myoglobin. It was the first protein whose structure they determined.
In 1958, Max Perutz and John Kendrew determined the 3D structure of Myoglobin by X-ray crystallography.
In 1962, a share of the Nobel Prize for Chemistry was awarded to John C. Kendrew for work using the X-ray diffraction technique that allowed the construction of a three-dimensional model of crystalline sperm-whale myoglobin.
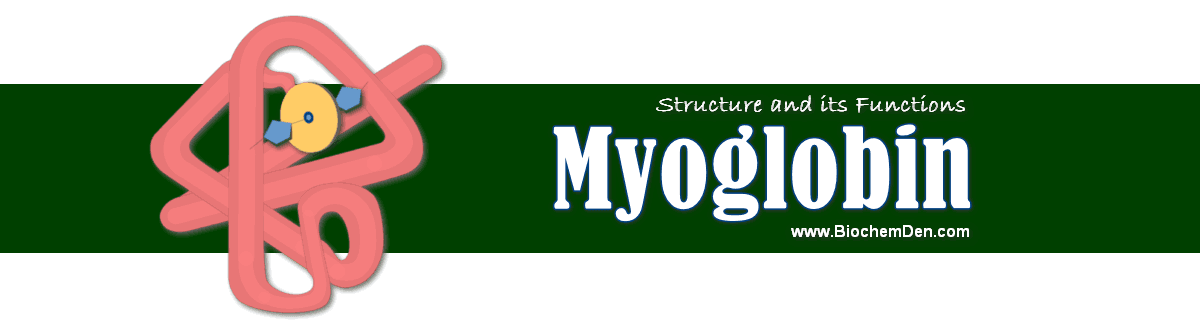
Myoglobin Structure
How is the structure of myoglobin related to its function?
Here is the basic information about Mb. It is a low molecular weight oxygen-binding heme protein.
This protein is mainly found in the heart and skeletal muscle cells and occurs in the highest concentration in the striated muscles of vertebrates.
- Myoglobin (myoG-Muscle; globinG=a type of protein) is a relatively small, oxygen-binding heme protein found in muscle cells.
- It is a monomeric protein that has 153 amino acid residues.
- It comprises eight α-helix connected through the turns with an oxygen binding site.
- Out of 153 amino acids, 121 (79%) are present in the helical regions, and they distribute the remaining 32 amino acids over the non-helical areas.
- It has a globular structure.
- Myoglobin has a molecular weight of 16,700, a compact macromolecule with an oblate, spheroid-shaped macromolecule.
- The overall molecular dimensions are 45 × 35 × 25 Ao.
- It contains a heme (prosthetic) responsible for its primary function (carrying oxygen molecules to muscle tissues) and Globin protein.
- It is a protein found in muscles that binds oxygen with its heme ring-like hemoglobin.
- The heme group comprises a protoporphyrin organic component and an iron atom at its center.
- It gives muscles and blood their distinctive red color.
- The Mb, a protein that stores oxygen,
- Oxidation of the iron atom (Fe2+ → Fe3+) is mainly responsible for the color of muscle and blood.
- The prosthetic heme group binds to the proximal histidine group, while a distal histidine group interacts on the other side of the plane in the structure.
- Myoglobin without its heme prosthetic is designated apo-myoglobin. The primary function of apo-myoglobin is to provide a hydrophobic environment for the heme group.
- It is found in skeletal muscle and serves to transport oxygen from the cell membrane to the mitochondria.
Functions of myoglobin
- The Mb is found in the muscle cells of animals. It is a protein.
- It acts as an oxygen reservoir (or) oxygen storage unit. It supplies oxygen to working muscles.
- Diving mammals such as seals and whales can remain submerged for long periods of time because they have more significant amounts of myoglobin than other animals.
- What is unique about this protein? It allows the body to have its own blood supply, like hemoglobin.
- The myoglobin protein is found only in vertebrates, such as birds, reptiles, amphibians, or fish. It plays a part in storing oxygen at high concentrations inside muscle cells, which is needed for movement.
Myoglobin vs. Hemoglobin
Kindly let us know What is the difference between myoglobin and hemoglobin? There are several critical differences between myoglobin and hemoglobin.
- Myoglobin and hemoglobin are two types of proteins responsible for transporting oxygen in the body.
- Myoglobin is found in muscle tissue, and hemoglobin is found in red blood cells.
- Both myoglobin and hemoglobin have a heme group containing iron, giving them their red color.
- Myoglobin is a monomer, meaning it is a single protein molecule, while hemoglobin has two sets of dimers, meaning it comprises two groups of protein molecules.
- Hemoglobin is also found in higher concentrations in the blood than myoglobin.
- Myoglobin stores oxygen in muscle tissue, and hemoglobin is responsible for transporting oxygen throughout the body.
- When oxygen levels are low, myoglobin releases oxygen into the muscles, so they can continue to function.
- When oxygen levels are high, hemoglobin binds to oxygen and transports it to the tissues and organs that need it.
- The protein’s ability to bind and release oxygen is due to its ability to form a complex with oxygen, in which there are four atoms of iron (Fe2+) bound with three atoms of carbon (C).
Clinical importance of myoglobin
- Myoglobin is a non-specific marker that helps detect the presence of myocardial infarction (MI). Myoglobin causes blood levels to rise in the relatively early stages of MI.
- The Mb is a non-specific marker for this disease because it also grows with cardiac muscle injury and appears in non-life-threatening situations such as exercise stress, heavy exertion, or intense emotions.
- The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and may cause acute kidney injury.
- The increased myoglobin has a low specificity for acute myocardial infarction (AMI). Other tests such as CK-MB, cardiac troponin, ECG, and clinical symptoms should be used to diagnose.
Myoglobin blood test
A myoglobin blood test is used to measure the level of protein myoglobin in the blood. Myoglobin is a protein found in muscle tissue that helps store and transport oxygen in the blood.
The level of myoglobin in the blood can be affected by many factors, including exercise, diet, and certain medical conditions.
The myoglobin blood test can help diagnose muscle disorders, such as muscular dystrophy and monitor the level of myoglobin in the blood of people with these conditions.
Synthetic analogs of MyoGlobin
Picket fence Porphyrin is a model of myoglobin. Myoglobin is a protein that contains heme, and heme is the part of the molecule that involves electron transfer with O2.
Picket fence porphyrin was widely studied since it was very familiar to virtually anybody in the field who looked at it in specific contexts, particularly as an O2 complex model system involving di-ferric states.
The O2 substrate adopts a bent geometry, occupying the sixth position on each iron center.
Frequently Asked Questions (FAQs)
What is myoglobin protein?
Myoglobin is a globular protein found in the muscle cells of humans that helps in the storage and release of oxygen. This protein is present in the muscles, and it helps carry oxygen.
What is the role of myoglobin?
The role of myoglobin is Oxygen Storage. It facilitates respiration in rapidly respiring muscular tissues. The solubility of oxygen increases, and it stimulates the diffusion of oxygen.
What does myoglobin do in the human body?
Myoglobin is a protein found in the muscles of vertebrates and other organisms. It is a globular protein with a heme group at each end, making it an essential part of the respiratory chain.
What is the normal range for myoglobin?
The normal range of myoglobin is less than 90mcg/L.
What is the difference between myoglobin and hemoglobin?
It is a monomeric protein that is present in muscular cells. Hemoglobin is a tetrameric protein that is present in the blood. Myoglobin’s function is to store oxygen in myocytes—hemoglobin functions as a gas exchanger.
How does myoglobin damage the kidney?
When muscle tissue is damaged, large concentrations of myoglobin enter the kidneys. Mb is considered highly toxic when this happens and may contribute to acute renal failure.
How is myoglobin regulated in the human body?
Myoglobin is a protein found in the muscles that bind to oxygen. It is also called hemoglobin, and it helps carry oxygen from the lungs to the muscles. To understand how myoglobin is regulated in the human body, we must first understand how it works. The iron atom of myoglobin can bind with an oxygen molecule and release it when required. When there is too much oxygen available, myoglobin will remove some of it, and when there isn’t enough, it will bind more with the functional oxygen molecules.
What are the different types of myoglobin?
The four types of myoglobin are:
a. Type I is the most common type of myoglobin in humans.
b. Types II and III are found in fish and amphibians.
c. Type IV is found in plants.How is myoglobin distributed in the body?
Myoglobin is a protein found in the muscles of mammals that helps store oxygen and release it as needed. In humans, myoglobin is mainly found in muscle tissues and some organs. The distribution of myoglobin in the body depends on the type of muscle tissue or organ. For example, myoglobin is more concentrated in cardiac muscle than in skeletal muscle because cardiac muscle needs to be able to use oxygen quickly for its high-energy needs.
What is the subcellular location of myoglobin in myocytes?
The subcellular location of myoglobin in myocytes is the cytoplasmic.
Is myoglobin found in the mitochondria?
No, myoglobin is not found in the mitochondria. Myoglobin is a protein found in muscle cells, and it stores oxygen and releases it to the muscle cells when needed. Myoglobin can be found in all muscles, but it is most abundant in red meat. The mitochondria are organelles that produce energy for the cell, and they do not contain any myoglobin.
What color would hair if the follicles started producing myoglobin along with melanin?
The color of the hair would depend on the amount of myoglobin produced. If the follicles had more myoglobin than melanin, the hair would be red. If the follicles produce more melanin than myoglobin, the hair would be black.
What is myoglobin’s function in the kidneys?
Myoglobin is a protein found in the muscles and helps store oxygen and release it to the muscles during aerobic respiration. Myoglobin also functions as an oxygen carrier in the kidneys, and Myoglobin helps regulate renal blood flow and urine production in the kidneys. It also works with hemoglobin, which is found in red blood cells, to help maintain healthy oxygen levels in the body.
Summary of the Myoglobin
Myoglobin is an oxygen carrier protein found in muscle cells, where it binds molecular oxygen (O2) during periods of heavy exercise or stress. This protein is found in all types of muscle tissue, but it is especially abundant in cardiac and skeletal muscle. It is also found in other tissues, such as the liver, kidneys, and brain. Myoglobin is a heme-based protein, which means that it contains a porphyrin ring similar to the heme group in hemoglobin and chlorophyll.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.