Monosaccharides are the simplest carbohydrates in that they cannot be hydrolyzed to smaller carbohydrates. They are basic units of Carbohydrates. They are made up of only one carbohydrate moiety. The general chemical formula of an unmodified monosaccharide is (C•H2O) n, literally a “carbon hydrate”. This is termed as the empirical formula.
In this formula, the “n” varies from 3-6 and rarely seven. This implies that in nature no. of carbon atoms found in monosaccharide varies from minimum 3 to maximum 7.
The smallest monosaccharides (for which n = 3) are dihydroxyacetone and glyceraldehyde. However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates.
Monosaccharides Classification
- Placement of its carbonyl group,
- Number of carbon atoms it contains, and
- Its chiral handedness.
1. Placement of its carbonyl group
If the carbonyl group is an aldehyde and attached to C-1, the monosaccharide is an Aldose; if the carbonyl group is a ketone attached to C-2, the monosaccharide is a Ketose. Therefore monosaccharides are called aldoses and ketoses derivatives of polyhydric alcohols. Glucose is an example of an aldose and fructose is an example of ketose.
2. Number of Carbon Atoms
Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses, six are hexoses, and so on. These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone). Various examples of other monosaccharide are given in the following table.
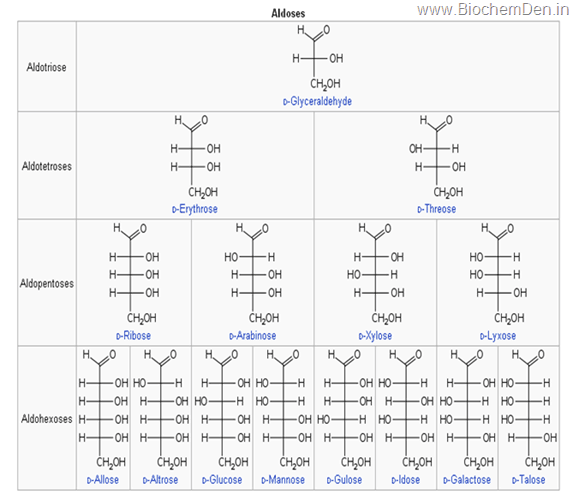
Trioses
A triose is a monosaccharide containing three carbon atoms. The general formula is C3H6O3. There are only two trioses, an aldotriose (glyceraldehyde) and a ketotriose (dihydroxyacetone). Trioses are important in respiration. Namely, lactic acid and pyruvic acid are derived from aldotriose and ketotriose, respectively.
Tetroses
A tetrose is a monosaccharide containing four carbon atoms. The general formula is C4H8O4. Example:D- Erythrose-4-P is an intermediate in hexosemonophosphate shunt which is an alternative of glucose oxidation
Pentoses
A pentose is a monosaccharide containing five carbon atoms. The general formula is C5H10O5.
Example of Pentoses are
- D- ribose is a constituent of RNA and many co-enzymes e.g. FAD, NAD.
- D-2 deoxy is a constituent of DNA component of DNA.
- D-Lyxose is a constituent of lyxoflavin found in the human heart.
- D- arabinose is a constituent of plant cell wall
- Phosphate esters of D- Ribulose and D- xylose occurs as an intermediate in the HMP pathway
Hexoses
A Hexose is a monosaccharide containing six carbon atoms. The general formula is C6H12O6
D- Glucose
It is the chief physiological sugar present in human blood. Its values are regulated between 70-110 mg/dl of blood by a pancreatic hormone Insulin and Glucagon. Increase in blood sugar levels leads to Diabetes. All tissues utilize glucose for energy. Brain and Erythrocytes depend exclusively on glucose. Its polymeric form glycogen is used as energy storage material in animals. Its polymeric form starch is used as energy storage material in plants.
D-Galactose
seldom found free in nature occurs as a constituent of milk sugar lactose and in tissues as galactolipids and glycoproteins.
D-Mannose
It is used to stamp proteins by the process of glucosylation. It does not occur free in nature but is widely distributed in combination as the polysaccharide mannan, e.g.-ivory nut. It is also found as the constituent of glycoproteins
D- Fructose
it is a ketohexose and is commonly called the fruit sugar, as it occurs in fruit. It is a sweet sugar sweeter than glucose and sucrose. It is found in honey as laevulose. In the seminal fluid of man fructose is the chief source of energy for sperms.
Heptoses
A heptose is a monosaccharide containing seven carbon atoms. The general formula is C7H14O7. Examples are Sedoheptulose It is a keto-heptulose found in plants of the sedum family. (most of the aldoses end in “-oses” and ketoses end in “-uloses” e.g. erythrose and erythrulose).
3. Its chiral handedness/ Isomerism
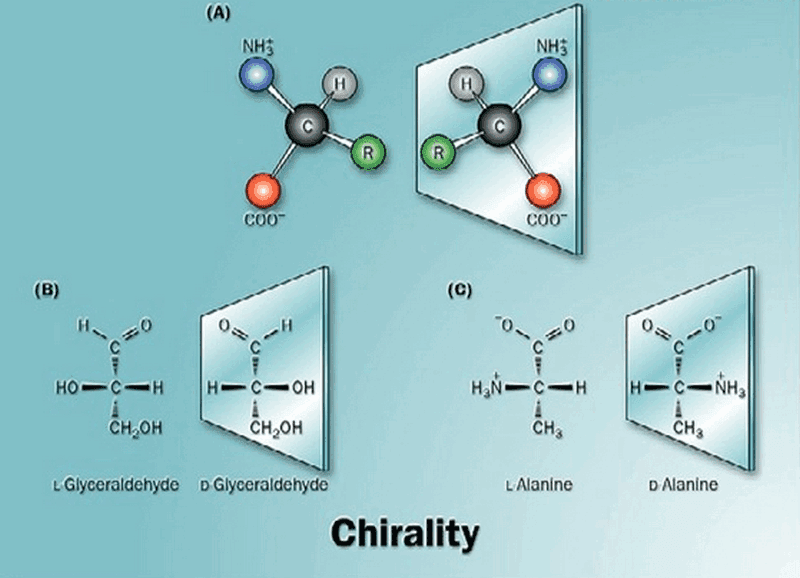
A chiral carbon is an asymmetric carbon which is linked to four different groups of molecules. Presence of asymmetric carbon gives rise to isomers within the same compound.
Isomers are molecules with same molecular formula but different structural formula.
All the monosaccharides except dihydroxyacetone contain one or more asymmetric (chiral) carbon atom. Thus most of the monosaccharides exhibit isomerism.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.