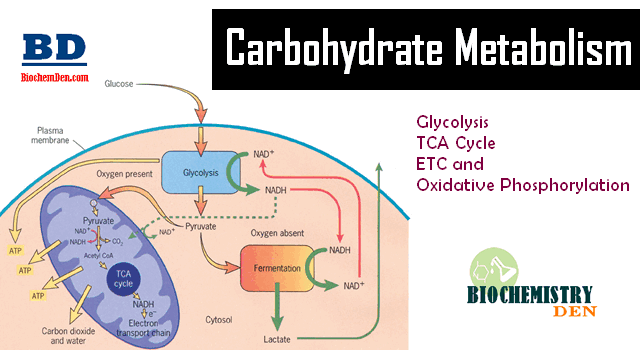
The coupling of oxidation with phosphorylation is termed “Oxidative phosphorylation”, which takes place during the electron transport in the inner membrane.

DEFINITION:
What is the definition of Oxidative Phosphorylation. The Endergonic synthesis of ATP from ADP and pi in mitochondria is called “Oxidative phosporylation”, which is catalyzed by the enzyme “ATP synthase” (or) “mitochondrial ATPase “ (or) “H+-ATPase” because it was discovered through its catalysis of hydrolytic reaction.
Hypothesis on Oxidative Phosphorylation:
Many hypothesis have been formulates to explain the coupling of electron – transport and ATP synthesis. But some are discussed below
(1) Chemical coupling hypothesis:
It was proposed by “Edward Slater” & “Lehninger (1967)”. According to this synthesis electron transport yields reactive intermediates whose break down drives oxidative phosphorylation.
Eg: In Glycolysis, oxidation of Gly-3-P by NAD+ gives 1,3-bis P glycerate. Its phosphate group is transferred from Enzyme intermediate to ADP.
The difficulty with this mechanism is no appropriate reactive intermediate have been identified.
So, the hypothesis not agreed by so many scientists.
(2) Conformational coupling hypothesis:
It was formed by “Poul boyer”. According to this hypothesis, electron transport cause4s activation of proteins of inner mitochondrial membrane. These activated proteins in some way associated with ‘ATP synthase’. The retention of activate4d protein, drives the ATP synthesis. The disadvantage with this mechanism is, it has little experimental support.
(3) Chemiosmotic Hypothesis:
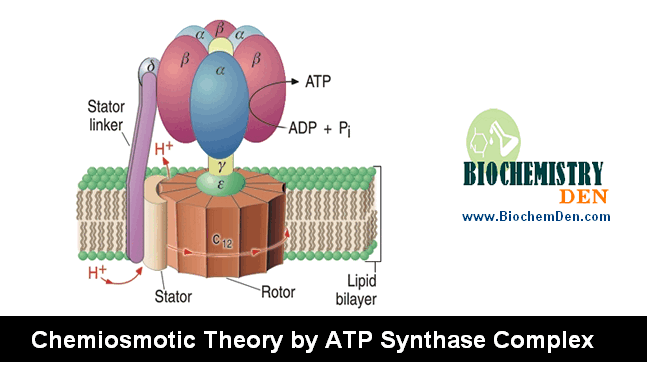
Peter Mitchell in 1961 (Nobel prize, 1978) proposed the chemiosmotic theory to explain the oxidative phosphorylation. “The transport of electrons from inside to outside of inner-mitochondrial membrane is accompanied by the generation of a proton gradient across the membrane. Protons accumulate outside the membrane, creating “electrochemical potential difference”. This proton motive force (pmf) drives the synthesis of ATP by ATP synthase complex.
Mitchell’s hypothesis, oxidative phosphorylations are coupled by a part on gradient is now supported by a wealth of evidence:
(a) Electron transport generates a proton gradient across the inner mitochondrial membrane. The PH outside is 1.4units lower than inside and the membrane potential is 0.14V, the outside being positive.
(b) ATP is synthesized when a PH gradient is impose4d on mitochondria in the absence of electron transport.
(c) NADH.Q reductase, Cyt. Reductase and Cyt.oxidse pump protons out of the matrix. Their return drives ATP formation by ATP synthase.
(d) A closed compartment is essential for oxidative phosphorylation. ATP synthesis coupled to electron transfer doesn’t occur in soluble preparations or in membrane4 fragments lacking well-defined inside and outside compartments.
Two theories established to explain the pumping of protons.
(i) Redox loop mechanism
(ii) Proton transport mechanism
(i) Redox loop mechanism:
‘Mitchell’ has proposed a fantastic scheme which is based on the first that reducing on the fact that reducing equivalents are transferred as ‘H’ atoms by some of the electron carriers (such as “Fe-S” center and cytochromes). He opined that hydrogen carrying and electron carrying proteins alternate in the respiratory chain to form 3 “functional loops” called the “oxidation-reduction loops” (=O/R loops). Each loop corresponds functionally to the coupling sites I, II and III of the chemical hypothesis respectively. In each loop, 2 protons are carried out through the loop from Matrix to inter membrane space; the corresponding pair of electrons is then carried back from the outer to the inner surface of the membrane. Each pair of reducing equivalents provides the osmotic energy to make one mole of ATP.
(ii) Proton transfer mechanism:
In the electron transport, as envisaged by Peter Mitchell, the respiratory chain is folded into 3 oxidation-reduction (o/r) loops. It is assumed that the members of the respiratory chain are organized in the membrane to provide the necessary sidedness. Each pair of electrons transferred from NADH to oxygen causes 6 protons to be translocated from inside to the outside of the coupling membrane.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.