The pyruvate dehydrogenase complex is a vital enzyme complex that bridges glycolysis and the citric acid cycle by converting pyruvate into acetyl-CoA. It is located in the mitochondrial matrix, where it plays a crucial role in linking glycolysis to the citric acid cycle. Understanding the structure and function of pyruvate dehydrogenase is essential for students of biochemistry and molecular biology, as its activity is critical for cellular energy metabolism.
This article delves into the mechanisms, regulation, and clinical relevance of the pyruvate dehydrogenase complex, offering a comprehensive guide enriched with the latest biochemical insights.
Pyruvate Dehydrogenase and Its Complex
The pyruvate dehydrogenase (PDH) enzyme catalyzes the irreversible conversion of pyruvate into acetyl-CoA, a crucial step linking glycolysis to the citric acid cycle.
Enzymatic subunits of PDH Complex:
The pyruvate dehydrogenase complex (PDC) is a multienzyme assembly, often referred to as a dehydrogenase complexed, comprising multiple copies of three core enzymes. The E1 component, in mammals, is a tetramer with two alpha subunits and two beta subunits, whereas in bacteria, it can exist as a dimer.
- E1 (pyruvate decarboxylase),
- E2 (dihydrolipoamide acetyltransferase), and
- E3 (dihydrolipoamide dehydrogenase).
CoEnzymes of PDH complex:
- Thiamine pyrophosphate (TPP)
- Lipoic acid
- Flavin Adenine Dinucleotide (FAD)
- CoEnzyme~A
- Nicotinamide Adenine Dinucleotide (NAD+)
The pyruvate dehydrogenase reaction generates NADH and releases carbon dioxide (CO2), which are critical for energy production and metabolic regulation.
This enzyme complex is essential for energy production in aerobic organisms. Without proper PDH activity, cells cannot efficiently convert pyruvate into acetyl-coenzyme, leading to metabolic imbalances. High energy states inhibit PDH activity, whereas low energy states stimulate it.
Moreover, the regulation of pyruvate dehydrogenase kinase expression plays a pivotal role in controlling the activity of the pyruvate dehydrogenase complex, making it a key point of metabolic control.
What Is the Structure of the Pyruvate Dehydrogenase Complex?
The structure of the pyruvate dehydrogenase is highly organized, forming a cubic core composed of E2 subunits surrounded by E1 and E3 enzymes. This arrangement ensures substrate channeling, which enhances the efficiency of pyruvate decarboxylation.
The pyruvate dehydrogenase complex from bovine heart has been extensively studied, revealing a cubic core of the pyruvate with a highly ordered assembly that facilitates dehydrogenase activity.
Structurally, the complex contains multiple E1, E2, and E3 components, making it a true pyruvate dehydrogenase multienzyme complex.
This multienzyme complex ensures that pyruvate metabolism is tightly coordinated, reducing the likelihood of toxic intermediate accumulation.
Additionally, studies on yeast pyruvate decarboxylase have helped elucidate how the E1 subunit interacts with cofactors like thiamine pyrophosphate to catalyze reactions efficiently.
About Pyruvate Dehydrogenase Enzyme
- PDH is a multienzyme complex
- The molecular weight of the PDH complex in Escherichia coli is 48,00,000.
- PDH complex is located in the Matrix Space of Mitochondria of the erythrocytes in the cytoplasm of the prokaryotes.
- The Enzyme contains 3 enzymatic sub-units and 5 co-enzymes.
Mechanism of Pyruvate Dehydrogenase Complex
The mammalian pyruvate dehydrogenase complex (PDC) is a multifunctional enzyme that converts pyruvate into acetyl coenzyme A, linking glycolysis with the Krebs cycle. This reaction is essential for aerobic metabolism and oxidative metabolism, supporting energy production in healthy cells and tumor cells.
The structure of the human PDC is more complex than that of prokaryotic PDC, incorporating additional structural components like E3BP.
The complex is located at the mitochondrial membrane and consists of three catalytic enzymes (E1, E2, and E3) arranged with octahedral symmetry, forming a large catalytic machine.
The conversion of pyruvate into acetyl-CoA involves multistep reactions, and the process can be described in five main steps:
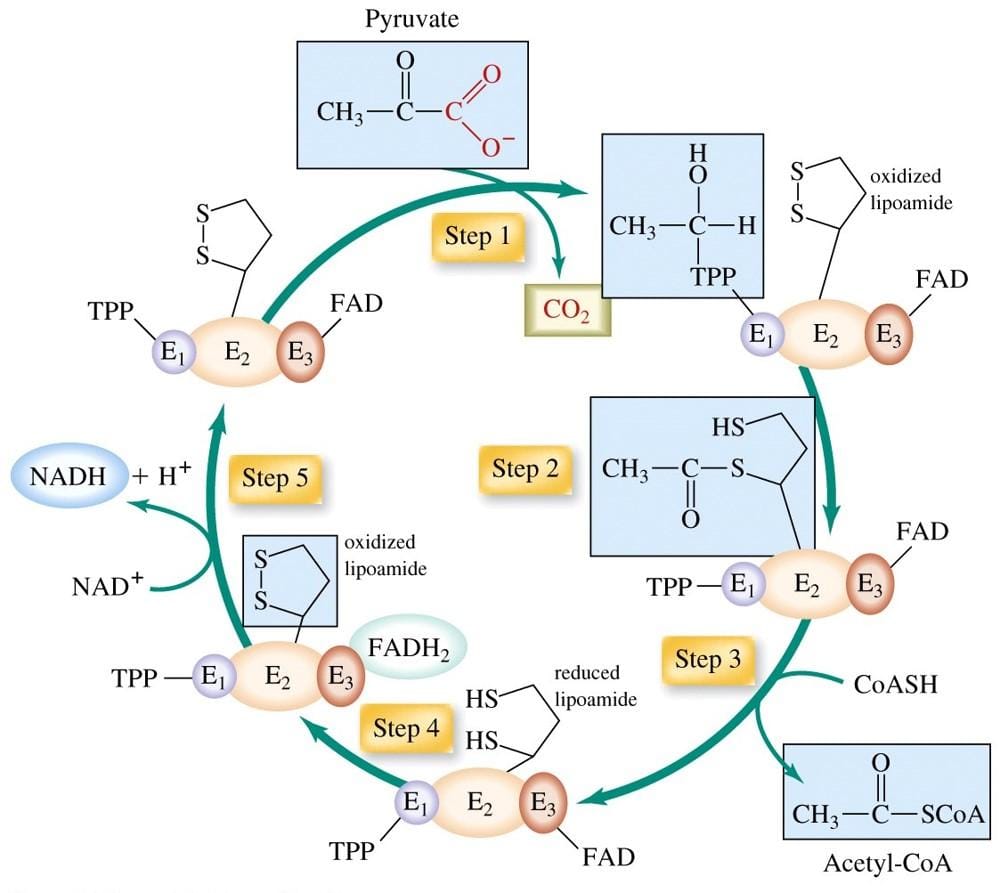
Step 1: Decarboxylation of Pyruvate
- Pyruvate enters the complex and interacts with the active site of the E1 catalytic enzyme.
- The reaction catalyzed by E1 removes a carboxyl group from pyruvate as CO₂, forming a hydroxyethyl intermediate bound to thiamine pyrophosphate (TPP).
- This step uses the alternating active site mechanism, ensuring efficient transfer of intermediates between active sites.
- The decarboxylation step is crucial for glucose homeostasis and energy supply.
Step 2: Oxidation
- The hydroxyethyl group is oxidized and transferred to the lipoamide arm of the E2 enzyme (dihydrolipoyl acetyltransferase).
- This forms an acetyl-thioester on the lipoamide, a process called reductive acetylation.
- The peripheral subunit binding domain helps position the lipoamide for proper transfer using electrostatic and hydrophobic interactions.
- This step prepares the acetyl group for transfer to Coenzyme A.
Step 3: Acetylation and Formation of Acetyl-CoA
- The acetyl group is transferred from the lipoamide to Coenzyme A, producing acetyl coenzyme A, which enters the Krebs cycle for further energy generation.
- The E2 enzyme contains catalytic domains that facilitate this catalytic process.
- The β subunit and other interaction loci help maintain the structure of the catalytic machine, ensuring smooth multistep reactions.
- This step links carbohydrate metabolism to fatty acids synthesis and energy production.
Step 4: Reduction of Lipoamide
- After transferring the acetyl group, the E2 lipoamide is fully reduced.
- The E3 enzyme (dihydrolipoyl dehydrogenase) oxidizes the lipoamide by transferring electrons to its FAD cofactor, regenerating the oxidized lipoamide.
- The reaction is guided by hydrophobic interactions and electrostatic interactions that stabilize binding sites between enzymes.
- This step ensures the catalytic sites are ready for the next round of reactions.
Step 5: Regeneration and NADH Formation
- Finally, electrons from FADH₂ of E3 are transferred to NAD⁺, forming NADH, which contributes to ATP production through the electron transport chain.
- This step completes the catalytic process, maintaining efficient oxidative metabolism and aerobic metabolism.
- Reversible phosphorylation by pyruvate dehydrogenase kinase and phosphatase regulates the activity of catalytic enzymes, allowing metabolic regulation in response to energy demands. The phosphorylation of PDH by PDK is influenced by the levels of acetyl-CoA and NADH.
- This regulation can be competitively inhibited in some conditions like cancer cells, affecting glucose homeostasis and promoting alternative energy pathways.
Key Features of the Mechanism
- The complex is a catalytic machine with multiple catalytic domains and active sites arranged in octahedral symmetry.
- Electrostatic and hydrophobic interactions stabilize enzyme-substrate binding and substrate channeling.
- The alternating active site mechanism ensures intermediates are efficiently transferred between three catalytic enzymes.
- Mutations or deficiencies (PDH deficiency) can result in metabolic disorders, poor feeding, or lactic acidosis in children.
- The mammalian pyruvate dehydrogenase complex is essential for both normal cells and tumor cells, where its activity affects oxidative metabolism and energy production.
- Structural evidence and studies like Arjunan et al provide insight into binding sites, catalytic sites, and interaction loci, critical for definitive diagnosis of PDC-related disorders.
How Does the E1 Component Function in Pyruvate Decarboxylation?
The E1 component, also called pyruvate decarboxylase, is the first enzyme in the pyruvate dehydrogenase complex. It catalyzes the decarboxylation of pyruvate into acetyl-CoA, a critical step for aerobic energy production. The e1 subunit binds pyruvate and cofactors, facilitating the oxidative decarboxylation of pyruvate.
In addition to E1, E3 (dihydrolipoamide dehydrogenase) interacts with the e1 and e3 components, ensuring that electrons are transferred efficiently, and the activity of the complex remains optimal. The complex e1 component is essential for the conversion of pyruvate to acetyl-CoA, making it a cornerstone of pyruvate metabolism and energy regulation in mitochondria.
Role of Pyruvate Dehydrogenase in Pyruvate Metabolism
The pyruvate dehydrogenase complex (PDC) plays a central role in pyruvate metabolism, connecting carbohydrates, fats, and amino acids to energy production. It acts as a bridge between glycolysis and the Krebs cycle by converting pyruvate into acetyl coenzyme A.
Key Functions
Conversion of Pyruvate:
- The complex carries out pyruvate decarboxylation, removing a carbon from pyruvate and linking it to coenzyme A.
- This produces acetyl-CoA, which enters the Krebs cycle to generate ATP during aerobic metabolism.
Energy Regulation:
- The activity of the pyruvate dehydrogenase complex ensures efficient energy production from glucose.
- Proper function prevents the buildup of pyruvate and lactate, which can cause metabolic imbalance.
Multienzyme Coordination:
- The pyruvate dehydrogenase multienzyme complex e1 works together with E2 and E3 enzymes in a multistep catalytic process.
- This allows smooth transfer of intermediates and maintains high efficiency through catalytic sites and active site interactions.
Regulation by Kinase and Phosphatase:
- Pyruvate dehydrogenase kinase (PDK) can inactivate the complex through reversible phosphorylation, slowing down oxidative metabolism when energy is sufficient.
- Pyruvate dehydrogenase phosphatase (PDP) reactivates the complex when energy is needed, ensuring glucose homeostasis.
Importance
- Supports the production of acetyl-CoA, which is a key substrate for the Krebs cycle and fatty acid synthesis.
- Maintains aerobic metabolism and energy balance in both normal cells and tumor cells.
- Dysregulation or PDH deficiency can lead to metabolic disorders, poor energy production, and accumulation of lactate.
Regulation of Pyruvate Dehydrogenase by Kinase and Phosphatase
The mammalian pyruvate dehydrogenase complex is a catalytic machine whose activity is tightly controlled to maintain glucose homeostasis and support aerobic metabolism. Its regulation occurs mainly through reversible phosphorylation by two enzymes: pyruvate dehydrogenase kinase (PDK) and pyruvate dehydrogenase phosphatase (PDP).
- Phosphorylation by PDH kinase:
- Inactivates the three catalytic enzymes of the complex.
- Reduces the oxidative metabolism of pyruvate into acetyl coenzyme A.
- Can be competitively inhibited under certain conditions, like in cancer cells, where energy needs are altered.
- Dephosphorylation by PDH phosphatase:
- Reactivates the complex, restoring catalytic process and decarboxylation of pyruvate into acetyl-CoA.
- Supports entry of pyruvate into the Krebs cycle for ATP production.
Factors Influencing PDH Regulation
- Energy levels:
- High ATP or NADH → activate PDH kinase, slowing the oxidation of pyruvate.
- High ADP or pyruvate → activate PDH phosphatase, enhancing catalytic activity.
- Hormones and nutrient signals:
- Insulin and other signals can modulate PDH kinase expression, adjusting metabolic regulation based on energy needs.
- Cell type differences:
- In tumor cells, PDH activity may be altered to favor glycolysis over oxidative metabolism, a phenomenon known as the Warburg effect.
- In normal cells, PDH ensures aerobic metabolism and proper energy supply.
Key Points to Remember
- The mammalian pyruvate dehydrogenase complex is regulated by reversible phosphorylation, balancing energy production.
- PDH kinase inactivates the complex, while PDH phosphatase reactivates it.
- Regulation is influenced by energy status, hormones, and nutrient availability, ensuring proper pyruvate metabolism.
- Dysregulation can contribute to PDH deficiency, poor glucose homeostasis, or altered metabolism in cancer cells.
Clinical Implications of Pyruvate Dehydrogenase Deficiency
Pyruvate dehydrogenase complex deficiency is a genetic disorder characterized by:
- Reduced pyruvate dehydrogenase activity leading to impaired conversion of pyruvate to acetyl-coa.
- Clinical manifestations including lactic acidosis, neurological deficits, and developmental delays.
- Possible presentation as Leigh syndrome, which involves neurodegeneration and lesions in the central nervous system.
- A wide spectrum of metabolic disturbances depending on the severity of the enzyme defect.
- Evidence from studies on 82 patients with pyruvate dehydrogenase complex mutations showing variations in the spectrum of defects, underscoring the critical role of human pyruvate dehydrogenase in energy metabolism.
- Treatment options such as ketogenic diet, thiamine supplementation, and administration of dichloroacetate aimed at bypassing the defective pathway or enhancing residual enzyme activity.
Enzyme Cofactors and Their Importance in Dehydrogenase Activity
The pyruvate dehydrogenase complex requires several cofactors for optimal function, including thiamine pyrophosphate (TPP), lipoamide, CoA, FAD, and NAD+. These cofactors facilitate pyruvate decarboxylation and electron transfer, ensuring the activity of the whole complex.
Dihydrolipoamide dehydrogenase acts in concert with cofactors to regenerate oxidized lipoamide, maintaining the oxidative decarboxylation of pyruvate. Deficiency of these cofactors can impair pyruvate dehydrogenase activity, leading to metabolic disorders, highlighting the importance of both enzyme structure and cofactor availability.
Variations in Human Pyruvate Dehydrogenase Complex
Human pyruvate dehydrogenase complex exhibits variations across tissues and developmental stages. Kidney pyruvate dehydrogenase complex, for instance, shows distinct regulatory patterns compared to liver or muscle, adapting to tissue-specific energy demands.
The protein of the human pyruvate and subunit of pyruvate dehydrogenase demonstrate diversity in sequence and activity, which can affect the activity of the pyruvate dehydrogenase complex. Understanding these variations is essential for interpreting metabolic studies and designing therapeutic interventions for disorders like complex deficiency or pyruvate dehydrogenase complex deficiency.
Summary of Most Important Things to Remember
- Pyruvate dehydrogenase complex bridges glycolysis and the citric acid cycle by converting pyruvate into acetyl-CoA.
- The E1 component catalyzes pyruvate decarboxylation, initiating the conversion process.
- Pyruvate dehydrogenase kinase inactivates, while pyruvate dehydrogenase phosphatase activates the complex, regulating pyruvate metabolism.
- Cofactors like TPP, lipoamide, FAD, and NAD+ are essential for dehydrogenase activity.
- Pyruvate dehydrogenase complex deficiency can cause metabolic and neurological disorders, highlighting clinical relevance.
- Variations in human pyruvate dehydrogenase complex reflect tissue-specific energy requirements.
- Proper function of the multienzyme complex ensures efficient energy production and prevents accumulation of toxic intermediates.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.