Carbohydrates are a class of organic molecules with the general chemical formula Cn(H2O)n. They are composed of carbon, hydrogen, and oxygen. Carbohydrates perform a large number of important roles within living organisms.
Some of their uses include fuel for cellular respiration, the main source of energy for living organisms, the storage of energy in the form of chemical bonds, and the structural component of some cells.
In this blog, we will discuss the color reactions of carbohydrates.
What Are the Color Reactions of Carbohydrates? Carbohydrates are a class of organic molecules with the general chemical formula Cn(H2O)n. These compounds are literally carbon hydrates of monomers.
There are a number of informative simple tests that may be used to characterize sugars. These tests will utilize a test reagent that will yield a colour change after reacting with specific functional groups of the compounds being tested.
These are reactions that can detect the presence or absence of carbohydrates in test solutions. They range in specificity from the very general to the very specific.
In this experiment, the Molisch test, Iodine test, Benedict’s test, Barfoed’s Test, Seliwanoff’s test and Bial’s Test were conducted to determine the presence of a particular carbonyl group.
Several qualitative tests have been devised to detect members of this biologically significant class of compounds.
These tests will utilize a test reagent that will yield a color change after reacting with specific functional groups of the compounds being tested.
The following exercises are reactions that can detect the presence or absence of carbohydrates in test solutions. They range in specificity from the very general (i.e., Molisch test for carbohydrates) to the very specific (i.e., mucic acid test for galactose).
You are given solutions containing: fructose, glucose, lactose, galactose, ribose, ribulose, sucrose, and starch. Devise a scheme by which you can systematically identify these compounds.
Introduction
Simple sugars, starches, and cellulose are organic compounds that have the approximate formula C(H2O)n, which accounts for the name carbohydrate or hydrate of carbon that is usually applied to this group of compounds.
They are not truly hydrates of carbon but are polyhydroxy (alcohol) compounds that contain an aldehyde or ketone, functional group.
These functional groups give the carbohydrates some of their chemical properties. Several qualitative tests have been devised to detect members of this biologically significant class of compounds.
These tests will utilize a test reagent that will yield a color change after reacting with specific functional groups of the compounds being tested.
These reactions can detect the presence or absence of carbohydrates in test solutions. They range in specificity from the very general to the very specific.
The Chemical Test and Procedure
| Test | Procedure |
| Iodine Test | + 10 drops iodine reagent, observe |
| Benedict’s | + 10 drops Benedict’s reagent, water bath (100 degrees Celsius) |
| Barfoed’s | + 10 drops Barfoed’s reagent, water bath (100 degrees Celsius) |
| Seliwanoff’s | + 10 drops Seliwanoff’s reagent, water bath (100 degrees Celsius) |
| Bial’s | + 10 drops of Bial’s reagent, water bath (100 degrees Celsius) |
Carbohydrates are found in fruits, vegetables, grains, beans, and dairy foods. They are important because they provide energy for our bodies. The color reactions chart illustrates how certain carbohydrates react in water or vinegar solutions.
- Cane sugar (glucose) has a blue color.
- Corn syrup is brownish orange.
- Fructose is yellow.
- Lactose is white. These colors indicate the type of foodstuff the carbohydrate comes from.
For example, lactose is milk sugar. It is found naturally in milk. If lactose was to be dissolved in a water solution, it would turn into a milky liquid. This reaction occurs when milk is heated and left to stand for a prolonged time at room temperature. Lactose is also called milk sugar.
What is the basic procedure for the color reaction of carbohydrates?
Perform the following qualitative tests on 0.2 M solutions (unless otherwise stated) of starch, sucrose, glucose, lactose, galactose, ribose, and ribulose. Use the scheme you devised in the prelab section to identify an unknown solution.
The unknown will be 1 of the above solutions or a mixture of 2 of the above solutions.
Test 1: Molisch’s Test for Carbohydrates
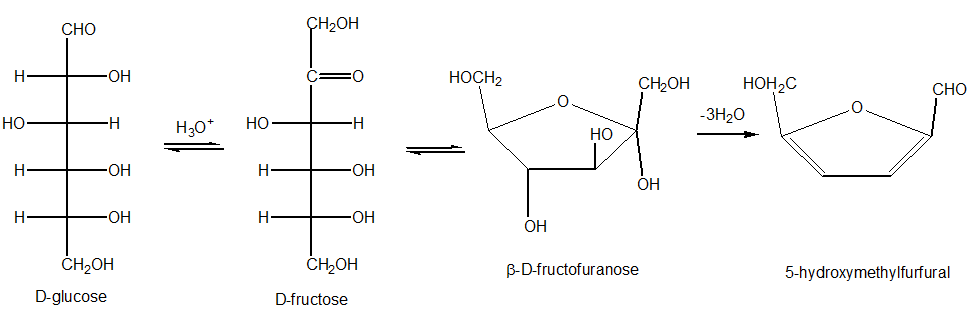
The Molisch test is a general test for the presence of carbohydrates. Molisch’s reagent is a solution of alpha-naphthol in 95% ethanol. This test is useful for identifying any compound that can be dehydrated to furfural or hydroxymethylfurfural in the presence of H2SO4.
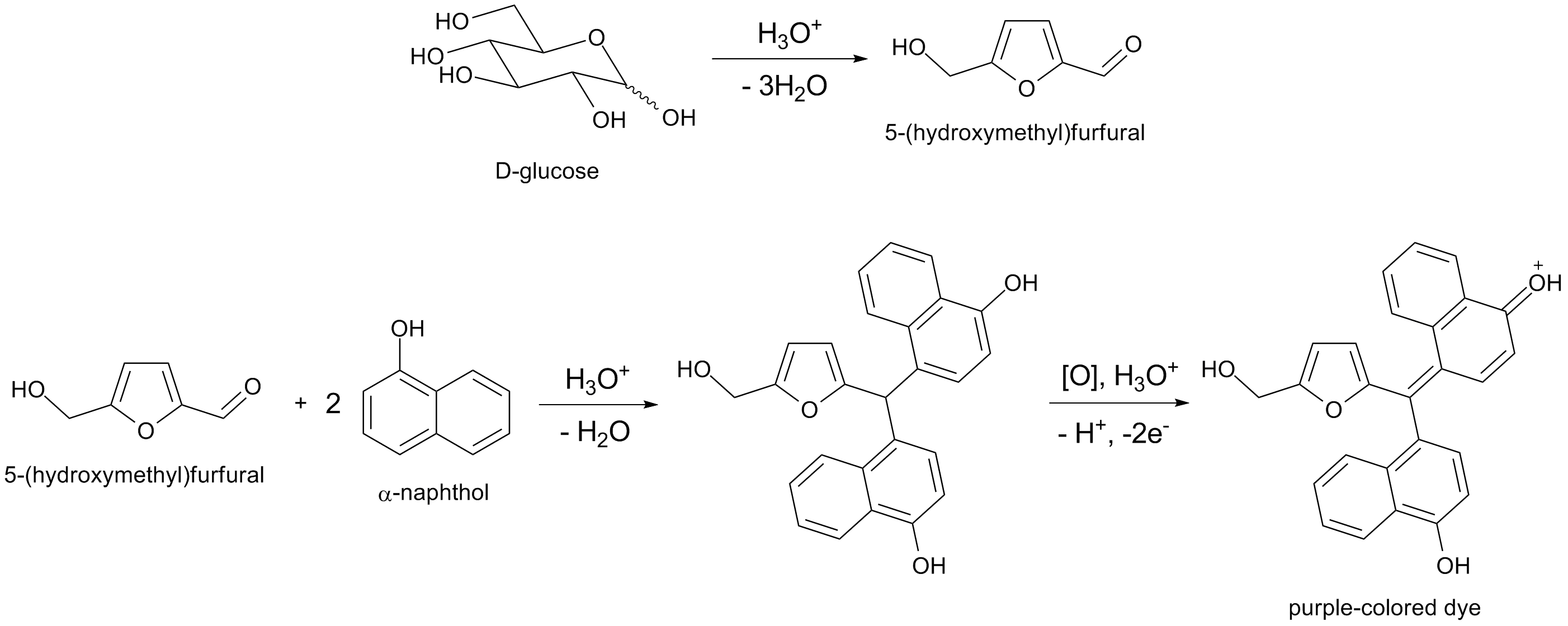
Furfural is derived from the dehydration of pentoses and pentosans, while hydroxymethylfurfural is produced from hexoses and hexosans. Oligosaccharides and polysaccharides are hydrolyzed to yield their repeating monomers by the acid.
The alpha-naphthol reacts with the cyclic aldehydes to form purple condensation products. Although this test will detect compounds other than carbohydrates (i.e., glycoproteins), a negative result indicates the absence of carbohydrates.
Method: Add 2 drops of Molisch reagent to 2 mL of the sugar solution and mix thoroughly. Incline the tube, and gently pour 5 mL of concentrated H2SO4 down the side of the test tube. A purple colour at the interface of the sugar and acid indicates a positive test. Disregard a green colour if it appears.
Test 2: Benedict’s Test for Reducing Sugars
Alkaline solutions of copper are reduced by sugars that have a free aldehyde or ketone group, with the formation of coloured cuprous oxide. Benedict’s solution is composed of copper sulfate, sodium carbonate, and sodium citrate (pH 10.5).
The citrate will form soluble complex ions with Cu++, preventing the precipitation of CuCO3 in alkaline solutions.
Method: Add 1 ml of the solution to be tested to 5 mL of Benedict’s solution, and shake each tube. Place the tube in a boiling water bath and heat for 3 minutes. Remove the tubes from the heat and allow them to cool. The formation of a green, red, or yellow precipitate is a positive test for reducing sugars.
Test 3: Barfoed’s Test for Monosaccharides
This reaction will detect reducing monosaccharides in the presence of disaccharides. This reagent uses copper ions to detect reducing sugars in an acidic solution. Barfoed’s reagent is copper acetate in dilute acetic acid (pH 4.6). Look for the same color changes as in Benedict’s test.
Method: Add 1 ml of the solution to be tested to 3 mL of freshly prepared Barfoed’s reagent. Place test tubes into a boiling water bath and heat for 3 minutes. Remove the tubes from the bath and allow them to cool.
The formation of a green, red, or yellow precipitate is a positive test for reducing monosaccharides. Do not heat the tubes longer than 3 minutes, as a positive test can be obtained with disaccharides if they are heated long enough.
Test 4: Lasker and Enkelwitz Test for Ketoses
The Lasker and Enkelwitz test utilize Benedict’s solution, although the reaction is carried out at a much lower temperature. The color changes that are seen during this test are the same as with Benedict’s solution. Use dilute sugar solutions with this test (0.02 M).
Method: Add 1 ml of the solution to be tested to 5 mL of Benedict’s solution to a test tube and mix well. The test tube is heated in a 55°C water bath for 10–20 minutes. Ketopentoses demonstrate a positive reaction within 10 minutes, while ketohexoses take about 20 minutes to react. Aldoses do not react positively to this test.
Test 5: Bial’s Test for Pentoses
Bial’s reagent uses orcinol, HCl, and FeCl3. Orcinol forms coloured condensation products with furfural generated by the dehydration of pentoses and pentosans. It is necessary to use dilute sugar solutions with this test (0.02 M).

Method: Add 2 ml of the solution to be tested to 5 mL of Bial’s reagent. Gently heat the tube to boiling. Allow the tube to cool. The formation of a green solution or precipitate denotes a positive reaction.
Test 6. Mucic Acid Test for Galactose
Oxidation of most monosaccharides by nitric acid yields soluble dicarboxylic acids. However, oxidation of galactose yields an insoluble mucic acid. Lactose will also yield a mucic acid, due to the hydrolysis of the glycosidic linkage between its glucose and galactose subunits.
Method: Add 1 mL of concentrated nitric acid to 5 ml of the solution to be tested and mix well. Heat on a boiling water bath until the volume of the solution is reduced to about 1 mL. Remove the mixture from the water bath and let it cool at room temperature overnight. The presence of insoluble crystals in the bottom of the tube indicates the presence of mucic acid.
Test 7. Iodine Test for Starch and Glycogen
The use of Lugol’s iodine reagent is useful to distinguish starch and glycogen from other polysaccharides. Lugol’s iodine yields a blue-black color in the presence of starch.
Glycogen reacts with Lugol’s reagent to produce a brown blue color. Other polysaccharides and monosaccharides yield no color change; the test solution remains the characteristic brown-yellow of the reagent.
It is thought that starch and glycogen form helical coils. Iodine atoms can then fit into the helices to form a starch-iodine or glycogen-iodine complex.
Starch is the form of amylose and amylopectin has fewer branches than glycogen. This means that the helices of starch are longer than glycogen, therefore binding more iodine atoms.
The result is that the colour produced by a starch-iodine complex is more intense than that obtained with a glycogen-iodine complex.
Method: Add 2–3 drops of Lugol’s iodine solution to 5 mL of the solution to be tested. Starch produces a blue-black color. A positive test for glycogen is a brown-blue color.
A negative test is the brown-yellow color of the test reagent.
- What are Polysaccharides and How it was Classified?
- What are the Classifications of Carbohydrates? Explain with Examples
- Carbohydrates are the Staff of Life. Why?
- What are Monosaccharides? How to Classify?
Positive Reactions for Carbohydrates Test
Test | Positive colour change |
Molisch | deep purple/ purple |
Iodine | black/blue black |
Benedict | rust-coloured |
| Barfoed | red-brick coloured precipitate |
| Seliwanoff | deep cherry red |
| Bial’s test | yellow-orange |
The reaction of Carbohydrate Samples
Samples | Molisch | Iodine | Benedict | Barfoed | Seliwanoff | Bial |
| Glucose | + | – | + | ++ | – | – |
| Fructose | ++ | – | + | ++ | ++ | ++ |
| Ribose | – | – | – | – | – | – |
| Lactose | + | – | + | – | – | – |
| Sucrose | ++ | – | – | – | – | ++ |
| Starch | ++ | ++ | – | – | – | – |
| Unknown 1 | + | – | ++ | ++ | ++ | ++ |
| Unknown 2 | + | – | + | – | – | – |
| Identity of Unknown 1: | Fructose | |||||
| Identity of Unknown 2: | Lactose |
Qualitative Analysis protocols
Quantitative Analysis protocols
- Assay of Acid Phosphatase enzyme activity from Potatoes
- Assay of Urease Enzyme Activity (Enzymology Practical Protocol)
- Effect of Temperature on Amylase activity (Enzymology Protocol)
- Assay of Salivary Amylase enzyme activity
- Determination of Carbohydrate by Anthrone Method
Legend: (++) = fast reaction/ (+ )=slow reaction/ (-) is no reaction
Frequently Asked Questions (FAQs)
What color change indicates the presence of glucose?
It turns yellow to Orange
What reagent is used to test for carbohydrates?
Benedict’s reagent is a test for the presence of many simple carbohydrates. When it reacts with reducing sugars, it changes color from turquoise to yellow or orange.
What color is a positive test for starch?
Adding iodine solution (yellow/brown) to starch and watching for a color change is a biochemical test for starch. Iodine turns a blue/black color when exposed to starch.
What is the general test for carbohydrates?
Benedict’s reagent is a test for the presence of many simple carbohydrates. When it interacts with lowering sugars, it changes color from turquoise to yellow or orange. These are unbound aldehyde or ketone groups in simple carbohydrates.
What color does iodine turn in the presence of glucose?
Iodine becomes blue/black when it comes into contact with starch. Using this iodine solution test, you can tell the difference between starch and glucose (and other carbs). A peeled potato, for example, will turn black if iodine is put to it. Glucose may be measured using Benedict’s reagent.
What reagent is used to test for reducing sugars?
Benedict’s solution, which is in excess (blue solution).
What color does glucose turn with an iodine test?
This test helps in distinguishing glucose from starch. It’s a physical challenge. Adding iodine solution (yellow/brown) to starch and watching for a color change is a chemical test for starch. Iodine turns a blue/black color when exposed to starch.
Why does iodine not react with glucose?
Iodine reacts with starch to produce a blue to black complex, but not with glucose. Inside the amylose coil, the iodine molecule slides. This forms a soluble linear triiodide ion complex that slides into the starch coil, resulting in an intense blue-black color.
Why do we add hydrochloric acid when testing for non-reducing sugars?
The hydrochloric acid breaks the sugar down into the individual monosaccharides that make it up – it breaks the glycosidic bonds between each monosaccharide.
Why does glycogen give a red color with iodine?
The iodine test is used to differentiate between starch, glycogen, and other saccharides. When iodine is added to a starch solution, it becomes blue. The blue hue is caused by iodine being trapped in the helical coils of amylose. Glycogen, on the other hand, becomes red when exposed to iodine.
Why does Benedict’s test require heat?
Heat is required to give the necessary energy for the reaction. All the cells in the human body require glucose as a source of energy. Blood glucose levels (blood sugar levels) should ideally be controlled within a relatively tight range.
Which of the following sugar gives a positive color reaction with the mucic acid test?
Galactose
Which color appears as the benedict’s reagent reacts?
Brick – Red Color
Cellulose gives which color with iodine?
When Cellulose reacts with iodine, gives Red color.
Which reagent is used to detect the presence of starch?
Iodine is used to detect the presence of starch.
Get this protocol in PDF format. Just download this “Color Reactions of Carbohydrates” file and make a print and distribute to the students. It helps you to protect your students from spelling mistakes and volumetric errors. All the best
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.