Heme is a Porphyrin molecule. The porphyrins found in nature are compounds in which various side chains are substituted for the 8 hydrogen atoms numbered in Porphin nucleus. These notes explain about Heme synthesis in animals and plants.
- What is the Composition of Blood?
- What happens to Blood Sugar after a Meal?
- Plasma Proteins: Types and Functions (Basic Notes)
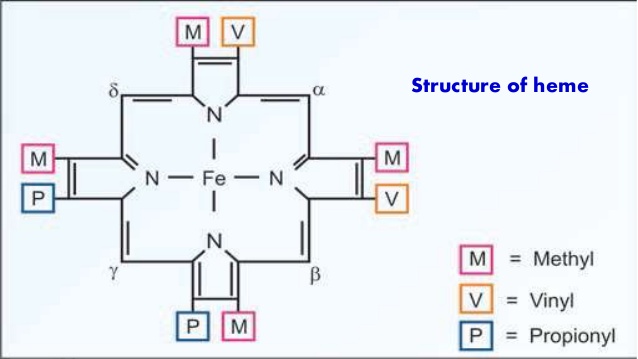
- Heme is the non-protein part mainly present in that mean which is one of the constituents of Hemoglobin, Chlorophyll, Myoglobin, and Cytochromes.
- Heme is a porphyrin nucleus which has a tetrapyrrole ring.
- In the structure of heme.
- It contains 4 pyrrole nucleus is connected by methylene bridges
- It is a planner molecule. The ring contains Vinyl groups(-CH=CH2), Methyl group (-CH3), Propionic acid groups (-CH2-CH2-COOH).
- The biosynthesis of heme mainly takes place partly in Mitochondria and partly in Cytosol of the liver
- The two major sites of heme biosynthesis are Erythroid cells, which synthesizes most of the remainder.
- The synthesis can be explained in several steps.

Steps of Heme synthesis
Both chlorophyll, the photosynthetic pigment of plants, and heme, the iron protoporphyrin of hemoglobin in animals are synthesized (Heme synthesis) in living cells by a common pathway.
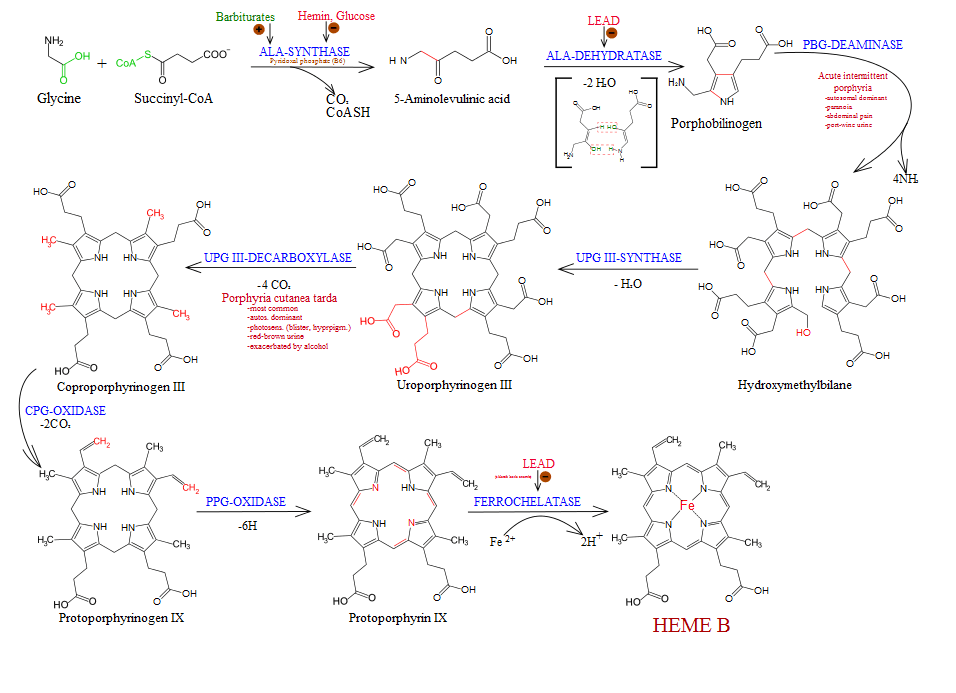
Step 1: Formation of δ-amino Levulinic acid (ALA):
The precursor molecule for the heme synthesis is simplest and non-essential and optically inactive amino acid Glycine and the TCA cycle intermediate Succinyl~coA enzyme. Glycine condenses with Succinyl~coA. It forms δ-amino Levulinic acid. This reaction catalyzed by ALA synthatase. This reaction takes place in mitochondria. This is the rate-limiting step of this pathway.
Glycine + Succinyl~coA → δ-amino Levulinic acid + CO2 + CoenzymeA
Step 2: Formation of Porphyrinogen:
This reaction takes place in Cytosol. The dehydration of two molecules of ALA to form Porphobilinogen by the enzyme ALA dehydrase. The enzyme is inhibited by heavy metal ion lead.
2 (δ-amino Levulinic acid) → Porphobilinogen + H2O
Step 3: Formation of Uroporphyrinogen:
The condensation of four molecules of porphyrinogen. It gives Uroporphyrinogen-III. This reaction catalyzes by Uroporphyrinogen-I synthatase and uroporphyrinogen-III cosynthatase takes place in the cytosol.
4 (Phorphobilinogen) →Uroporphyrinogen-III
Step 4: Formation of Heme:
UroPorphyrinogen-III is converted into Heme by a series of decarboxylation and oxidation. Finally the Uro-porphyrinogen-III converted into ProtoPorphyrinogen oxidase. The enzyme ProtoPorphyrin decarboxylase and protoporphyrin oxidase and protoporphyrinogen oxidase. The protoporphyrin-II is modified into heme by substituting the ferrous ion (Fe+3) by the enzyme Ferrochelation.
Uroporphyrinogen-III →→→ Protoporphyrin-IX + 6 Carbondioxide
Protoporphyrin-IX + Fe+3 →HEME
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.