Are you looking for the details of Inhibitors and UnCouplers of Oxidative Phosphorylation? here is the complete list. The ability to harness energy from external sources and utilize it for biological work is characteristic of all living organisms.

Phototrophs harvest the energy of light (Plants). Chemotrophs harvest energy from the oxidation of fuel molecules. The oxidation of foodstuffs occurs in three stages.
Oxidative Stages of Food Stuff
1. Primary Metabolism
In the first stage, DIGESTION in the gastrointestinal tract converts the macromolecules into small units. For example, proteins are digested to amino acids and Polysaccharides are into monosaccharides, etc. This is called Primary Metabolism.
2. Secondary Metabolism
The products of primary metabolism are absorbed, catabolized to smaller components and ultimately oxidized to carbon dioxide.
The reducing equivalents mainly generated in the mitochondria by the final common oxidative pathway, the citric acid cycle. In the final process, NADH and FADH2 are generated. This is called Secondary Metabolism.
3. Tertiary Metabolism (or) Internal Metabolism
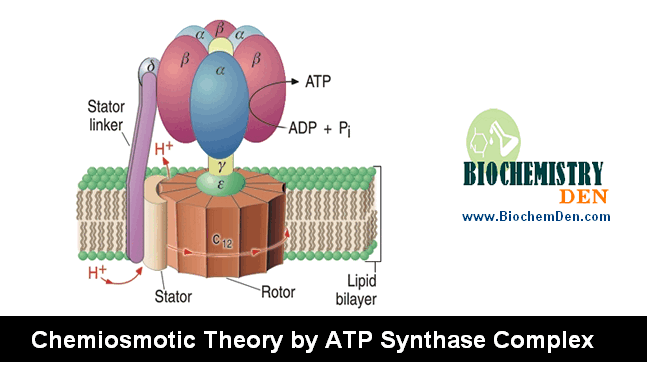
The secondary metabolism products Reducing equivalents (NADH and FADH2) enter into the electron transport chain (ETC, Respiratory chain), where energy is released.
This is called Tertiary metabolism (or) Internal Metabolism (or) Cellular Respiration.
Respiratory Inhibitors
The ETC chain contains 4 complexes (I, II, III and IV) that participate in the transfer of electrons. In general, it may be stated that the structural integrity of these complexes appears essential for its interaction with most inhibitors since the soluble, phospholipid free enzymes do not exhibit the characteristic inhibitory pattern.
The following compounds inhibit both electron transport and oxidative phosphorylation.
Inhibitors of Electron Transport
These are the inhibitors that arrest respiration by combining with members of the respiratory chain, rather than with the enzymes that may be involved in coupling respiration with ATP synthesis.
They appear to act at 3 loci that may be identical to the energy transfer sites I, II and III. The given below are the inhibitors of the Electron transport chain.
1. Rotenone
- It is the non-toxic inhibitors of Electron transport chain.
- These compounds extracted from roots of tropical plant Derris elliptica and Lonchoncarpus nicou.
- It binds at Complex I between Fe-S protein and Ubiquinone.
- This is non-toxic to mammals because poorly absorbed. Shows the toxic effect in fishes.
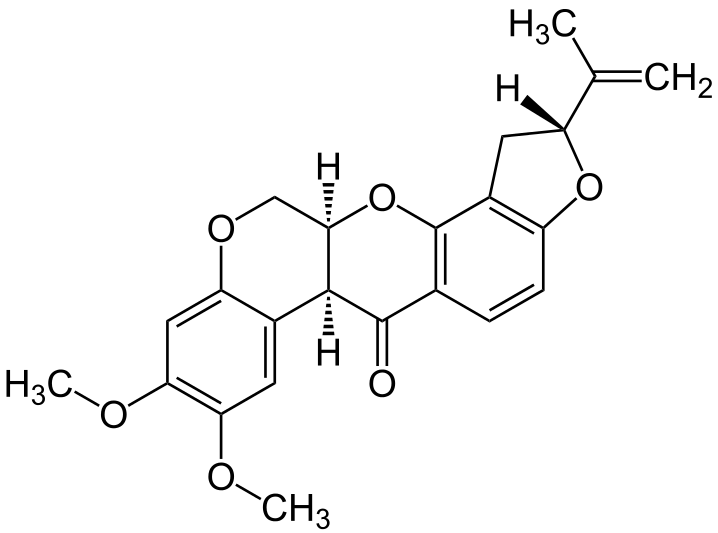
2. Piericidin A
- It is an Antibiotic.
- It is produced by species of streptomyces.
- The action is similar to Rotenone.
3. Barbiturates (Amytal, Seconal)
- It blocks NADH dehydrogenase and Coenzyme.Q
4. Antimycins
- Antimycin is the antibiotics, produced by Streptomyces. One of the inhibitors in the Electron transport chain.
- IT inhibits around site II and block electron flow between cytochromes b and c1, which prevents ATP synthesis coupled to the generation of a proton gradient. at site II.
- About 0.07 micromole of antimycin A per gram of mitochondrial protein is effective.
5. Dimercaprol
- It is identical in action to the antimycins.
6. Cyanides
- The cyanide ion (CN–) combines tightly with cytochrome oxidase, leading to
7. Azide
- Azide blocks the electron flow between the cytochrome oxidase complex and oxygen.
- Azide reacts with the ferric form (Fe3+) of this carrier.
8. Hydrogen Sulfide
- H2S is toxic, with disagreeing odor gives a warning.
- It inhibits Cytochrome Oxidase.
9. Carbon Monoxide
- It blocks cytochrome oxidase and Oxygen.
- It inhibits Fe2+
Inhibitors of Oxidative Phosphorylation
The given below is the list of inhibitors in Oxidative Phosphorylation.
1. Oligomycins
- It is a polypeptide antibiotic is obtained from various species of “Streptomyces”
- They inhibit the transfer of high-energy phosphate to ADP and also inhibit electron transfers coupled to phosphorylation.
- The antibiotic is a potent inhibitor to the ATP synthase complex.
2. Rutamycin
- This antibiotic also inhibits both ETC and oxidative phosphorylation.
3. Atractylate
- It backs oxidative phosphorylation by compelling with ATP & ADP for a site on the ADP-ATP antiport of the mitochondrial membranes. One of the inhibitors list which blocks the oxidative phosphorylation.
4. Bongkrekate
- It is a toxin formed by bacteria (Pseudomonas) in a coconut preparation from Java.1
- It also blocks the ADP-ATP antiport.
Uncouplers of Oxidative Phosphorylation
Uncouplers can be defined as A substance that uncouples phosphorylation of ADP from electron transfer.
Uncoupling agents are compounds which dissociate the synthesis of ATP from the transport of electrons through the cytochrome system. This means that the electron transport continues to function, leading to oxygen consumption but phosphorylation of ADP is inhibited.
Below are few uncoupling agents,
1. 2,4-Dinitrophenol
- A classic uncoupler of oxidative phosphorylation.
- The substance carries protons across the inner mitochondria membrane.
- In the presence of these uncouplers, electron transport from NADH to O2 proceeds normally, but ATP is not formed by the mitochondria. ATP is because the proton motive force across the inner mitochondrial membrane is dissipated.
- DNA and other uncouplers are very useful in metabolic studies because of their specific effect on outside phosphorylation.
2. Dicoumarol (Vitamin.K analog)
- Used as an anticoagulant.
3. Calcium
Transport of Ca+2 ion into mitochondria can cause uncoupling.
- Mitochondrial transport of Ca+2 is energetically coupled to oxidative phosphorylation.
- It is coupled with uptake of pi
- When calcium is transported into mitochondria, electron transport can proceed but energy is required to pump the4 Ca+2 into the mitochondria. Hence, no energy is stored as ATP.
4. CCCP (Chloro carbonyl cyanide phenyl hydrazone)
- Most active uncoupler
- These lipid-soluble substances can carry protons across the inner mitochondrial membrane.
5. Physiological un-couplers
- Excessive thyroxin hormone
- EFA deficiency
- Long-chain FA in brown adipose tissue
- Unconjugated hyperbilirubinemia
6. Valinomycin
- This is an example of an Ionophore of oxidative phosphorylation.
- Produced by a type of streptomyces
- It is a repeating macrocyclic molecule made up of four kinds of residues (L-lactate, L-Valine, D-hydroxyisovalarate, and D-Valine) taken 3 times.
- Transports K+ from the cytosol into matrix and H+ from the matrix to the cytosol, thereby decreasing the proton gradient.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.