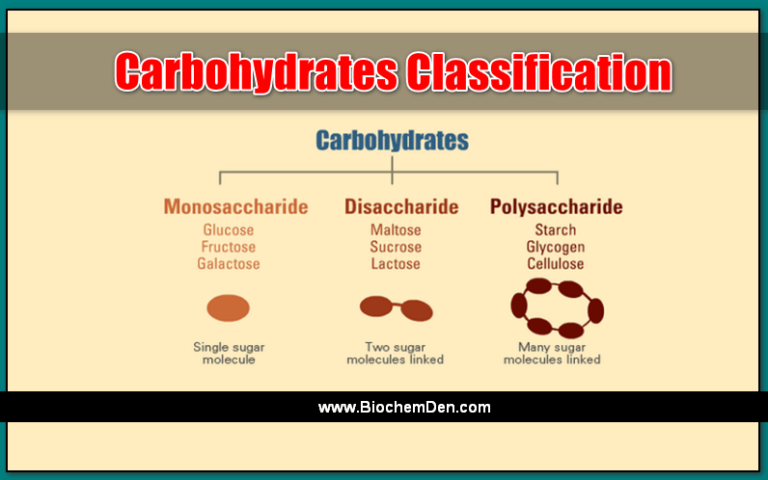
Mutarotation is the process by which the anomeric form of a monosaccharide, such as glucose, interconverts between its two forms, α and β. This spontaneous process occurs when the cyclic hemiacetal or hemiketal ring opens and the hydroxyl group at C1 changes configuration.
Understanding mutarotation and the equilibrium between α and β anomers is important for studying carbohydrate chemistry and metabolism.
Overview of Mutarotation
Monosaccharides with a chiral center at C1, such as glucose, can exist in two anomeric forms: α and β. This is because the hydroxyl group on C1 can be positioned either below (α) or above (β) the plane of the pyranose ring.
In D-glucose, the α form has the OH group in the axial position, while the β form has the OH group in the equatorial position. The two forms are diastereoisomers—epimers that differ in configuration only at C1.
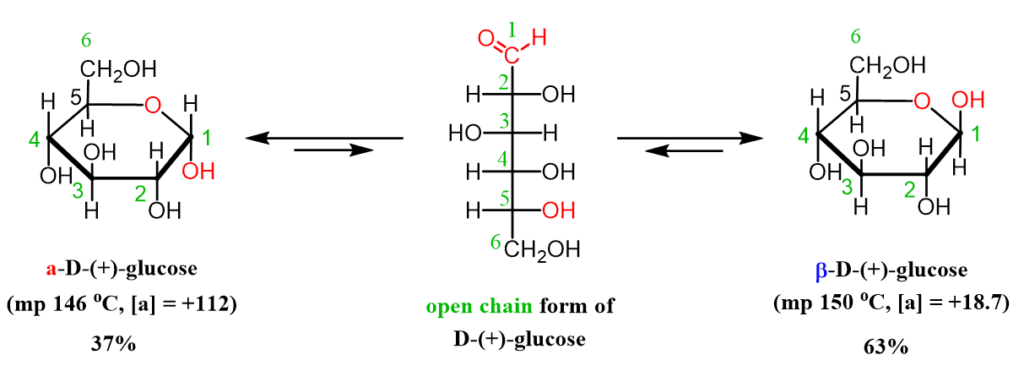
Mutarotation involves the interconversion between these α and β anomers when the hemiacetal or hemiketal ring momentarily opens. This opens up the possibility for the OH group at C1 to change configuration and close again in the opposite anomeric form.
This mutarotation process reaches an equilibrium mixture where both α and β anomers are present. The ratio of α to β at equilibrium is specific to each monosaccharide. For D-glucose, the equilibrium mixture contains about 64% β-D-glucopyranose and 36% α-D-glucopyranose.
Acids and bases both catalyze mutarotation, which can aid in the ring opening. It occurs spontaneously in aqueous solutions. By contrast, in crystalline form or nonpolar solvents, monosaccharides predominantly exist in the most thermodynamically stable anomeric form.
Kinetic and Thermodynamic Factors
The mutarotation process involves both kinetic and thermodynamic factors. While one anomer is typically more thermodynamically stable, the kinetic stability of the cyclic form means that both anomers co-exist.
The β anomer is typically more thermodynamically stable due to the axial orientation of the bulky anomeric OH group in the α form. This leads to unfavorable steric interactions. By contrast, the equatorial orientation of the OH in the β anomer experiences less steric strain.
However, the cyclic hemiacetal forms are more kinetically stable than the open-chain aldehyde. This kinetic stability means that both α and β anomers persist until equilibrium is reached between the two forms.
Their relative thermodynamic stability at equilibrium is what determines the ratio of anomers. The mutarotation process thus involves an interplay between kinetic and thermodynamic factors.
Mechanism of Mutarotation
The mechanism of mutarotation proceeds through the following steps:
- Ring-opening: The cyclic hemiacetal or hemiketal ring opens to give the open-chain aldehyde or ketone form.
- Tautomerization: Proton transfer occurs between the oxygen of the aldehyde or ketone and an adjacent hydroxyl group. This forms an enediol intermediate.
- Configuration change: The OH group at C1 shifts above or below the plane, changing between the α and β orientations.
- Ring-closing: The chain closes again to reform the cyclic hemiacetal/hemiketal in the opposite anomeric form.
This opening, tautomerization, flipping of the hydroxyl at C1, and reclosing allow for interconversion between α and β forms until equilibrium is reached.
Acidic or basic conditions facilitate the ring-opening step. Specific acid-base catalysis accelerates mutarotation by catalyzing the formation of the open-chain form.
Factors Affecting Mutarotation
Several factors affect the rate and equilibrium point of mutarotation:
- Temperature: Increasing temperatures accelerate mutation. Higher temperatures provide more energy to overcome the kinetic barrier of ring opening.
- pH: Low pH speeds up mutation since acidic conditions catalyze ring opening. High pH slows mutarotation.
- Solvent: Polar protic solvents like water facilitate mutarotation. Nonpolar solvents inhibit it.
- Structure – The specific monosaccharide structure affects the equilibrium anomeric ratio based on stereoelectronic factors.
- Concentration: At lower monosaccharide concentrations, mutarotation is faster since the open-chain form is not stabilized through hydrogen bonding with other monosaccharide molecules.
By understanding how these factors impact mutarotation kinetics and equilibria, we can better control this process in carbohydrate chemistry.
Monitoring Mutarotation
There are several techniques for monitoring the progress of mutarotation over time:
- Polarimetry: The α and β anomers have different specific rotations, so a polarimeter can follow the changing optical rotation as mutarotation proceeds.
- NMR spectroscopy, 1H NMR can distinguish between α and β forms based on the chemical shift and coupling of the anomeric proton. The ratio can be quantified over time.
- Chromatographic methods: HPLC can separate α and β anomers based on differences in retention time. Their peak areas denote the ratio.
- IR spectroscopy: The vibrational modes of α and β forms differ in the anomeric region, allowing IR to distinguish between them.
- Titration: Since the anomers have slightly different acid-base properties, pH titration curves track the changing ratio.
Kinetic parameters like the mutarotation rate constant and activation energy can also be derived from such measurements. These techniques help elucidate the mutarotation mechanism.
Biological Importance
Mutarotation plays an important biological role in carbohydrate metabolism.
- Isomerases catalyze mutarotation for rapid equilibration. This provides the proper anomer for subsequent enzymatic reactions.
- Mutarotation enables the cyclic conformations needed for transport across membranes.
- It facilitates alternating cyclic-open transitions during glycolysis.
- The equilibrium mixture presents both anomers for recognition by carbohydrate-binding proteins.
- Mutarotation forms reducing sugars with open-chain aldehyde or ketone groups necessary for Maillard reactions.
- It enables equilibrium between furanose and pyranose forms of pentose sugars like ribose and deoxyribose.
- Because the β anomers are more thermodynamically stable, they are more likely to form β-glycosidic bonds in biological systems.
Overall, mutarotation makes it possible for anomeric forms to change balance over time, which is important for the complex chemistry of carbohydrates in living things. Elucidating the mutarotation pathway for various sugars helps explain their biological fate and function.
Applications and Significance
In addition to its biological relevance, mutarotation has several practical applications:
- It impacts quality control and shelf life in the food industry. Mutarotation of glucose affects sweetness in sugars.
- The change in optical rotation during honey adulteration is used to detect dilution.
- Mutarotation rates help verify the authenticity and origin of honey samples.
- Monitoring mutarotation detects changes during fruit ripening.
- It is important for optimizing conditions in industrial fermentations.
- Mutarotation affects the production of high-fructose corn syrup.
- Molecular clock methods have used variable mutarotation rates for carbon dating archeological artifacts.
Overall, mutarotation is a key process underlying the chemistry of carbohydrates. Elucidating the kinetics and determining optimal conditions allows better control over sugar conformation and reactivity.
Key Terms Related to Mutarotation
- Anomer: One of two stereoisomers of a cyclic saccharide, differing in configuration only at the hemiacetal or hemiketal carbon (C1).
- Epimer: stereoisomers that differ in the configuration at only one chiral center. α and β anomers of sugars are epimers.
- Hemiacetal: A compound containing a hydroxyl group bonded to a carbon atom that is also doubly bonded to an oxygen atom. Formed when aldehydes react with alcohol groups.
- Hemiketal: The equivalent of a hemiacetal but with a ketone instead of an aldehyde.
- Glycosidic bonds: There is an O-glycosidic link between the anomeric carbon of one sugar and a hydroxyl group of the other sugar that connects the two of them.
- Pyranose: a cyclic monosaccharide form containing a six-membered ring structure analogous to pyran.
- Furanose: a cyclic monosaccharide form containing a five-membered ring analogous to furan.
- Tautomerization: It is a chemical reaction that changes the isomers of a compound so that a hydrogen atom can move from one atom to another. This creates different tautomeric structures.
Frequently Asked Questions about Mutarotation
What is mutarotation?
Mutarotation is the process where the alpha and beta anomeric forms of carbohydrates, like glucose, interconvert with each other by opening and closing the cyclic hemiacetal ring. This enables the hydroxyl group at C1 to change configuration and produce a different anomer.
Why does mutarotation occur?
Mutarotation occurs because monosaccharides can exist in equilibrium between the open-chain and cyclic structures. In the open-chain form, the hydroxyl at C1 is free to change from axial (alpha) to equatorial (beta) orientation. Reclosing into the cyclic structure then gives the opposite anomer.
What is the mechanism of mutarotation?
The process starts with opening the ring to let the aldehyde or ketone in. Then, tautomerization happens to make an enediol intermediate. Next, the C1 hydroxyl group is moved from the axial to the equatorial position or the other way around. Finally, the ring is closed again to create the cyclic hemiacetal or hemiketal.
What factors affect the rate of mutarotation?
Temperature, pH, solvent, concentration, and the specific monosaccharide structure all affect the mutarotation rate. Higher temperatures, acidic pH, polar protic solvents like water, and lower concentrations all speed up mutarotation.
How can you monitor the progress of mutarotation?
As mutarotation moves toward equilibrium, different techniques like polarimetry, NMR spectroscopy, IR spectroscopy, HPLC chromatography, and pH titrations can be used to track how the ratio of alpha to beta anomers changes over time.
Why is mutarotation important biologically?
Mutarotation lets cyclic and open forms of sugars change into each other, which is needed for transport across membranes, glycolysis, and enzyme reactions. It also facilitates anomeric equilibrium, which provides the proper anomer for binding and recognition by proteins.
How is mutarotation relevant industrially and commercially?
The rate of mutarotation and equilibrium need to be controlled in processes like making high-fructose corn syrup and fermentations. It also impacts the quality and shelf life of sugars in the food industry.
What are the major anomers of D-glucose?
The two major anomers of D-glucose are alpha-D-glucopyranose and beta-D-glucopyranose. Alpha has the hydroxyl group in the axial position, while beta has the equatorial hydroxyl. At equilibrium, beta-D-glucose predominates at about 64% over alpha.
How does mutarotation enable the tautomerization of the enediol intermediate?
Mutarotation allows the carbonyl oxygen to take a proton from the OH group next to it, opening the ring. This makes enediol, which is made up of C=C-C-OH. This shifts the double bonds and enables the hydroxyl at C1 to rotate into a different position.
Can mutarotation occur in sugars other than glucose?
Yes, mutarotation is a general phenomenon in sugars. Mannose, galactose, xylose, and ribose are some other monosaccharides that can change between alpha and beta-cyclic hemiacetals in the same way by opening and closing their rings.
Why does beta-D-glucose predominate at equilibrium?
The beta anomer predominates because it is more thermodynamically stable. The bulky hydroxyl group in the alpha anomer is oriented along the axis, which causes more steric strain. This makes the equatorial beta anomer less energetic when it is in equilibrium.
How does mutarotation allow sugars to act as reducing agents?
If you swap electrons with an aldehyde or ketone group, the open-chain form can act as a reducing agent after mutation. The free carbonyl group also enables reactions like glycosylation and Maillard browning reactions.
Can oligosaccharides and polysaccharides undergo mutarotation?
There are mutarotation monosaccharides in oligosaccharides and polysaccharides, but the ring cannot open because of the glycosidic bonds that hold the sugar units together. So oligosaccharides and polysaccharides do not typically undergo overall mutarotation.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.