Proteins are very important in biological systems as control and structural elements. Control functions of proteins are carried out by enzymes and proteinaceous hormones. Enzymes are chemicals that act as organic catalysts (a catalyst is a chemical that promotes but is not changed by a chemical reaction).
What are Proteins?
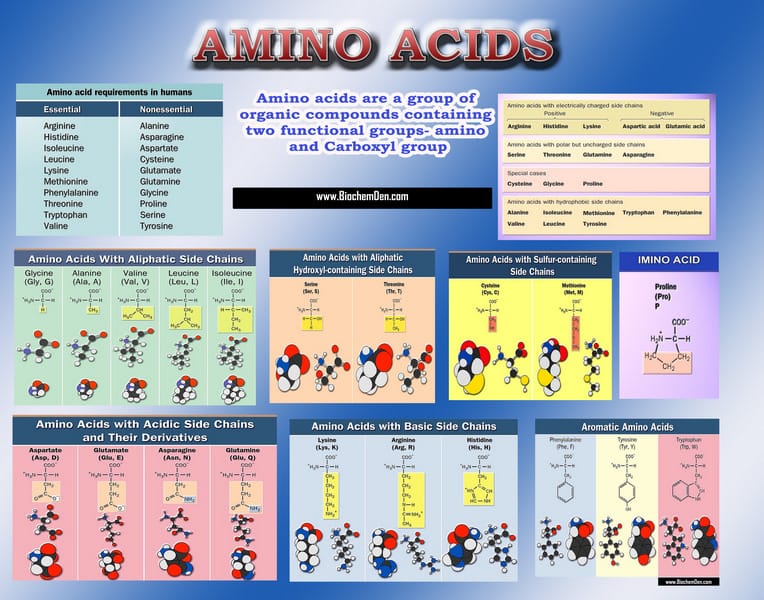
The building block of any protein is the amino acid, which has an amino end (NH2) and a carboxyl end (COOH). The structure of generalized amino acid, as well as the specific structures of the 20 biological amino acids, are shown in Figure 1 and 2 respectively.
The R indicates the variable component (R-group) of each amino acid. Alanine and Valine, for example, are both nonpolar amino acids, but they differ, as do all amino acids, by the composition of their R-groups. All living things (and even viruses) use various combinations of the same twenty amino acids. A very powerful bit of evidence for the phylogenetic connection of all living things.
Primary and Special Bonding in Proteins
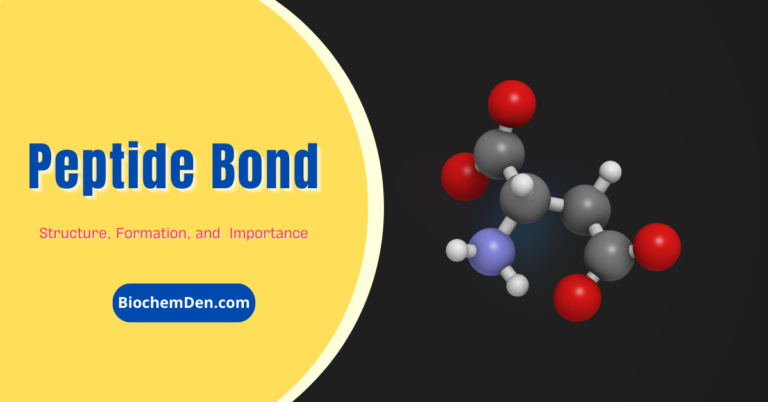
Amino acids are linked together by joining the amino end of one molecule to the carboxyl end of another. Removal of water allows the formation of a type of covalent bond known as a peptide bond. This process is illustrated in Figure 3.
- The peptide bond is the backbone of the proteins. Why?
- What are the stabilizing forces involved in Protein organizations
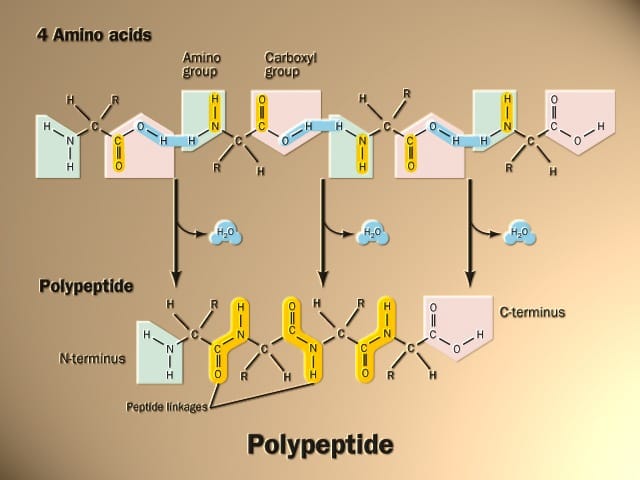
Amino acids are linked together into a polypeptide, the primary structure in the organization of proteins.
The primary structure of a protein is the sequence of amino acids, which is directly related to the sequence of information in the RNA molecule, which in turn is a copy of the information in the DNA molecule.
Changes in the primary structure can alter the proper functioning of the protein. Protein function is usually tied to their three-dimensional structure. The primary structure is the sequence of amino acids in a polypeptide.
The secondary structure is the tendency of the polypeptide to coil or pleat due to H-bonding between R-groups. The tertiary structure is controlled by bonding (or in some cases repulsion) between R-groups.
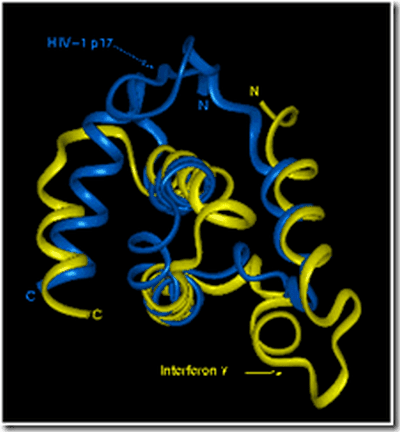
The tertiary structure of an HIV protein and its similarity to gamma interferon is shown in Figure 4. Many proteins, such as haemoglobin, are formed from one or more polypeptides. Such structure is termed Quaternary structure. Structural proteins, such as collagen, have regular repeated primary structures.
Like structural carbohydrates, the components determine the final shape and ultimately function.
- Collagens have a variety of functions in living things, such as the tendons, hide, and corneas of a cow.
- Keratin is another structural protein. It is found in fingernails, feathers, hair, and rhinoceros horns.
- Microtubules, important in cell division and structures of flagella and cilia (among other things), are composed of globular structural proteins.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.