Have you ever heard the term “Zwitterion” or “Isoelectric point” and wondered what they mean? If yes, you are in the right place. In this article, we will explain in detail what zwitterion and isoelectric point are, their properties, and their applications. So, let’s dive in.
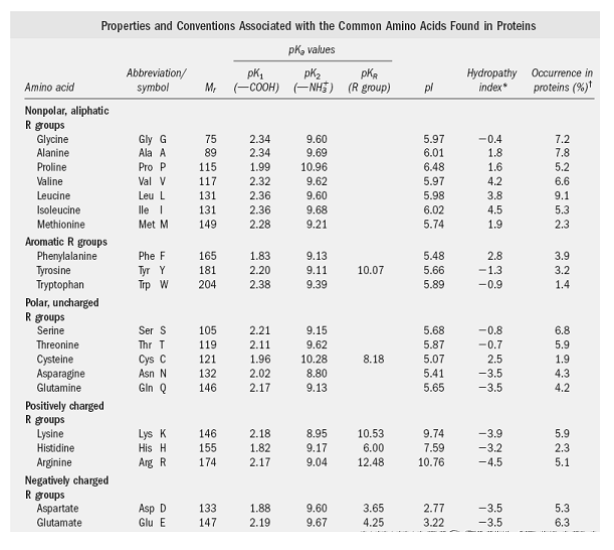
Amino acids have at least two ionizing groups, i.e., -COOH and -NH3+, of which the former dissociates more easily than the latter. At physiological pH (7.3), the carboxyl group exists in ionized form, whereas the amino group remains undissociated and thus retains a positive charge.

This doubly charged amino acid molecule with a negatively and positively charged group is electrically neutral and is known as a Zwitter ion.
What is Zwitter ion?
zwitterions, also known as dipolar ions, are chemical species containing positive and negative charges within the same molecule. In other words, a zwitterion has both acidic and primary functional groups.
Because of this, it can act as both an acid and a base in a chemical reaction. The word “Zwitter” comes from the German word for “hybrid” or “mixed.”
In strong acid conditions (around pH 1.1), the alpha-COOH group remains undissociated. However, when the pH is raised and reaches around pH 3.0, the proton from the carboxyl group leaves the COO group. This is called the pK of the acid group, and at this pH, dissociated and undissociated species are found in equal concentrations.
Similarly, when the pH is further raised to around 9.8, the amino group is dissociated – this pH is called the pK of an amino group. Thus, dicarboxylic acids like glutamic acid can have three pK values (two for carboxyl groups and one for the amino group) and four types of charged molecules, as shown below.
The name “zwitter” is derived from the German word “hybrid.”. Zwitterion (or) dipolar ion) is a hybrid molecule containing positively and negatively charged ionic groups. The proton shifts from the carboxyl group of the self-molecule to the amino group at normal pH cellular levels.
The amino acids rarely exist in a neutral form with free carboxyl (-COOH) and free amino (-NH2) groups. In strongly acidic pH (low pH), the amino acid is positively charged (Cation). At a specific pH, each amino acid carries both positive and negative charges and exists as a Zwitter ion.
What is an isoelectric point (pI)
The pH at which amino acids do not migrate towards the cathode (or anode) in the presence of an electric field is referred to as the “isoelectric point.”
The isoelectric point (pI) is the pH at which a molecule has a net charge of zero. At this point, the molecule is electrically neutral and does not migrate in an electric field. The pI is an essential property of a molecule as it affects its solubility and behavior in various applications.
Isoelectric pH is defined as the pH which a molecule exists as a zwitterion (or) dipolar ion and carries no net charge. Thus, the molecule is electrically neutral, but the charge will cancel each other.
Isoelectric Points of Some Important Proteins
- Isoelectric point of lysozyme (Egg white) – 11.0
- Isoelectric point of Insulin (Bovine) – 5.4
- Isoelectric point of Myoglobin (Horse) – 7.0
- Isoelectric point of Serum Albumin (Human) – 4.9
- Isoelectric point of Ribonuclease (Bovine) 7.8 / 9.5
Properties of zwitterions
Here are some properties of zwitterions:
- They contain both positive and negative charges within the same molecule.
- They are electrically neutral in solution.
- They have both acidic and primary functional groups.
- They are amphoteric, meaning they can act as both an acid and a base in a chemical reaction.
Examples of zwitterions
Here are some examples of zwitterions:
- Amino acids: Amino acids are the building blocks of proteins. They contain an amino group (-NH2) and a carboxyl group (-COOH) in the same molecule, making them zwitterions.
- Glycine: Glycine is the simplest amino acid and a Zwitter ion.
- Betaine is a naturally occurring compound found in sugar beets and other plants. It is also a Zwitter ion.
Properties of Isoelectric Point
Here are some properties of the isoelectric point:
- It is the pH at which a molecule has zero net charges.
- Above the pI, the molecule carries a net negative amount; below the pI, it carries a net positive charge.
- The pI is a function of the molecule’s amino acid sequence and 3D structure.
- The pI affects the solubility and behavior of a molecule in various applications.
Solubility and buffering capacity will be minimum at isoelectric pH
- To such a solution if we add hydrochloric acid drop by drop, at a particular pH, 50% of the molecules are in cationic form and 50% in zwitterionic form. Thus, pH is pK1 (with regard to –COOH). If more HCl is added, more molecules become cationic in nature and solubility increases.
- If we titrate the solution from pI with NaOH, molecules acquire the anionic form. When 50% of molecules are anionic that pH is called pK2.
- The isoelectric point (pI) for more amino, monocarboxylic amino acids can be calculated:
pK1 + pK2
pI =—————-
2
- The isoelectric point (pI) for more Acidic amino acids can be calculated:
pK1 + pK3
pI =—————-
2
- The isoelectric point (pI) for more amino, monocarboxylic amino acids can be calculated:
pK2 + pK3
pI =—————-
2
- pK1=Dissociation constant of (-COOH) group
- pK2=Association constant of (-NH2) group
- pK3=Dissociation (or) Association constant of “R” side chain
Applications of zwitterions and Isoelectric Point
Here are some applications of zwitterions and isoelectric points:
- Separation of amino acids: Ion exchange chromatography uses the isoelectric point to separate amino acids.
- Buffer solutions: zwitterions are used in buffer solutions as they can resist changes in pH.
- Biomolecules: zwitterions are commonly found in biomolecules such as proteins and peptides.
- Drug design: The isoelectric point is an essential property in drug design as it affects the solubility and stability of drugs.
Problems and Solutions on zwitterionand Isoelectric Point
1. Calculate the Isoelectric point of Glycine? (pK1=2.4; pK2=9.8)
Ans: Glycine is a neutral and optically inactive amino acid. According to isoelectric point concept, the given formula will be applied.
pK1 + pK2 2.4 + 9.8
pI =—————- = —————— = 6.1
2 2
The isoelectric point of the Glycine is 6.1
2. Calculate the Isoelectric point of Lysine? (pK1 = 2.2, pK2=8.9; pK3=10.5)
Ans: Lysine is basic amino acids (diamino monocarboxylic acid). According to isoelectric point concept, the given formula will be applied:
pK1 + pK3 2.2 + 10.5
pI =—————- = ————– = 9.7
2 2
The isoelectric point of the Lysine is 9.7
Lysine has Guanidinium group in the ‘R’ side chain of Lysine.
Frequently Asked Questions (FAQs):
What do you mean by zwitterion?
A zwitterion is a molecule that contains both a positive and a negative charge, resulting in a neutral overall charge.
Is zwitterion an acid or base?
A zwitterion can act as both an acid and a base depending on the context, as it has both acidic and basic functional groups.
How is the isoelectric point used in protein purification?
The isoelectric point is used in protein purification by ion exchange chromatography. This technique separates proteins based on their net charge at a particular pH. Proteins are loaded onto a column containing an ion exchange resin that is selectively charged. The proteins with a net charge opposite to that of the resin bind to the column and can be eluted with a salt gradient or pH change.
Are zwitterions and dipolar ions the same thing?
Yes, zwitterions and dipolar ions are the same things. They both refer to chemical species with positive and negative charges within the same molecule.
Can zwitterions act as both acids and bases?
zwitterions can act as both acids and bases in a chemical reaction. They have both acidic and primary functional groups within the same molecule. As a result, they can donate a proton (act as a base) to a molecule with a higher affinity for electrons or accept a proton (act as an acid) from a molecule with a lower affinity for electrons. This property makes zwitterions amphoteric and essential in various chemical and biological applications.
Is zwitterion always at pH 7?
Zwitterions are not always at pH 7; their specific pH can vary depending on the type of zwitterion and the surrounding conditions.
Why are amino acids called zwitterions?
Amino acids are called zwitterions because they contain both a positively charged amino group and a negatively charged carboxyl group, resulting in a neutral zwitterionic form.
Conclusion
In conclusion, zwitterions and isoelectric points are essential concepts in chemistry and biochemistry. zwitterions contain positive and negative charges within the same molecule and can act as both an acid and a base in a chemical reaction.
The isoelectric point is the pH at which a molecule has a net charge of zero and affects the solubility and behaviour of a molecule in various applications. Understanding these concepts is crucial in multiple fields, including biochemistry, drug design, and materials science.
Source: Dipolar molecules and it stages
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.