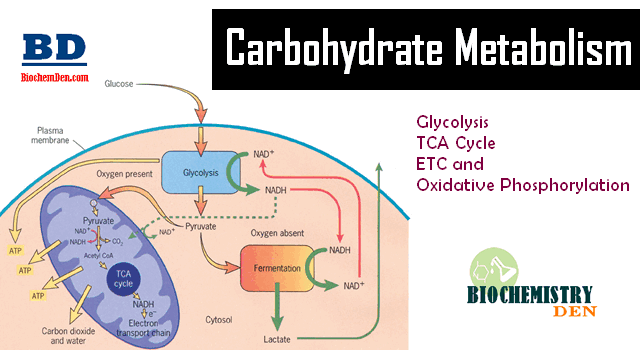
Carbohydrate metabolism denotes the various biochemical processes responsible for the formation, breakdown and interconversion of carbohydrates in living organisms. The most important carbohydrate is glucose, a simple sugar (monosaccharide) that is metabolized by nearly all known organisms. Since all digestible forms of carbohydrates are eventually transformed into glucose, it is important to consider how glucose is able to provide energy in the form of Adenosine triphosphate (ATP) to various cells and tissues. Glucose is metabolized in three stages in carbohydrate metabolism.Understanding the pathways of Carbohydrate Metabolism is essential for biochemistry students. 📖
They are…
- Glycolysis
- Oxidative phosphorylation and ETC
1. Glycolysis
During exercise, hormonal levels shift and this disruption of homeostasis alters the metabolism of glucose and other energy-bearing molecules. The breakdown of glucose to provide energy begins with glycolysis. To begin with, glucose enters the cytosol of the cell, or the fluid inside the cell not including cellular organelles.
- Carbohydrates are the Staff of Life. Why?
- Glycolysis – Glucose Catabolic Pathway
- Estimation of Blood Glucose level by Folin-Wu method
Next, glucose is converted into two, three-carbon molecules of pyruvate through a series of ten different reactions.
- A specific enzyme catalyzes each reaction along the way and a total of two ATP are generated per glucose molecule.
- Since ADP is converted to ATP during the breakdown of the substrate glucose, the process is known as substrate-level phosphorylation.
- During the sixth reaction, glyceraldehyde 3-phosphate is oxidized to 1,3 bisphosphoglycerate while reducing nicotinamide adenosine dinucleotide (NAD) to NADH, the reduced form of the compound.
- NADH is then shuttled to the mitochondria of the cell where it is used in the electron transport chain to generate ATP via oxidative phosphorylation.
- The most important enzyme in glycolysis is called phosphofructokinase (PFK)and catalyzes the third reaction in the sequence. Since this reaction is so favorable under physiologic conditions, it is known as the “committed step” in glycolysis. In other words, glucose will be completely degraded to pyruvate after this reaction has taken place.
- With this in mind, PFK seems as if it would be an excellent site of control for glucose metabolism. In fact, this is exactly the case.
When ATP or energy is plentiful in the cell, PFK is inhibited and the breakdown of glucose for energy slows down.Therefore, PFK can regulate the degradation of glucose to match the energy needs of the cell. This type of regulation is a recurring theme in biochemistry.
2.Krebs Cycle
Kreb’s Cycle is the central metabolic cycle of the Carbohydrate metabolism and all metabolic pathways. There are many compounds that are formed and recycled during the Krebs Cycle (Citirc Acid Cycle). These include oxidized forms of Nictotinamide adenine dinucleotide (NAD+) and Flavin adenine dinucleotide (FAD) and their reduced counterparts: NADH and FADH2. NAD+ and FAD are electron acceptors and become reduced while the substrates in the Krebs Cycle become oxidized and surrender their electrons.
The Krebs Cycle begins when the pyruvate formed in the cytoplasm of the cell during glycolysis is transferred to the mitochondria, where most of the energy inherent in glucose is extracted. In the mitochondria, pyruvate is converted to acetyl CoA by the enzyme pyruvate carboxlase.
In general, Acetyl-CoA condenses with a four carbon compound called oxaloacetate to form a six carbon acid. This six-carbon compound is degraded to a five and four carbon compound, releasing two molecules of carbon dioxide. At the same time, two molecules of NADH are formed.
Finally, the C-4 carbon skeleton undergoes three additional reactions in which guanosine triphosphate (GTP), FADH2 and NADH are formed, thereby regenerating oxaloacetate. FADH2 and NADH are passed on to the electron transport chain (see below) that is embedded in the inner mitochondria membrane.
- Citric acid cycle : Central metabolic cycle and its Significance
- Glycogenolysis : How Glycogen is Utilizing in Animals
- Glycogenesis : How to Synthesize Glycogen
3. Oxidative Phosphorylation / Electron Transport Chain:
GTP is a high-energy compound that is used to regenerate ATP from ADP. Therefore, the main purpose of the Krebs Cycle is to provide high-energy electrons in the form of FADH2 and NADH to be passed onward to the electron transport chain.
The high-energy electrons contained in NADH and FADH2 are passed on to a series of enzyme complexes in the mitochondrial membrane.
Three complexes work in sequence to harvest the energy in NADH and FADH2 and convert it to ATP: NADH-Q reductase, cytochrome reductase and cytochrome oxidase. The final electron acceptor in the electron transport chain is oxygen. Each successive complex is at lower energy than the former so that each can accept electrons and effectively oxidize the higher energy species.
In effect, each complex harvests the energy in these electrons to pump protons across the inner mitochondria membrane, thereby creating a proton gradient. In turn, this electro-potential energy is converted to chemical energy by allowing proton flux back down its chemical gradient and through specific proton channels that synthesize ATP from ADP.
- What is Mitochondria in Biological Sciences
- Electron Transport Chain Components in Mitochondria
- Electron Transport Chain Mechanism in Mitochondria
- Chemiosmotic Theory by ATP Synthase Complex
4. Hormonal Regulation of Carbohydrate Metabolism
Carbohydrate metabolism is tightly controlled by hormonal regulation, which is essential for maintaining blood glucose homeostasis throughout the body.
Pancreatic hormones, particularly insulin and glucagon, function as the primary regulators of glucose metabolism and work together to keep blood glucose levels within a narrow, physiological range.
The intricate interplay of these hormonal control mechanisms ensures that energy is available when needed while preventing dangerously high or low blood glucose levels.
Understanding the hormonal control of glucose metabolism is crucial for comprehending how the body maintains metabolic balance during both fed and fasted states.
a. Insulin and Glucagon: The Primary Regulators
Insulin is a peptide hormone secreted by beta (β) cells of the pancreatic islets of Langerhans, and it functions as the dominant anabolic hormone that reduces blood glucose levels during the fed state.
When blood glucose levels rise following a meal, insulin secretion increases and exerts its metabolic effects by promoting glucose uptake into cells and stimulating the synthesis of glucose storage compounds like glycogen and fat. I
nsulin regulates the overall metabolism of carbohydrates by activating glycolytic enzymes and inhibiting gluconeogenesis, thereby lowering circulating glucose concentrations.
This anabolic action of insulin ensures that excess glucose is efficiently stored for later use.
Conversely, glucagon is secreted by alpha (α) cells of the pancreatic islets and functions as the primary counter-regulatory hormone that increases blood glucose levels during the fasting state.
Glucagon stimulates catabolic processes including glycogenolysis (the breakdown of glycogen into glucose) and gluconeogenesis (the synthesis of new glucose), which work together to maintain adequate blood glucose levels when dietary carbohydrates are unavailable.
The opposing actions of insulin and glucagon create a balanced system of metabolic hormone fluctuations that maintains blood glucose homeostasis.
b. Counter-Regulatory Hormones: Additional Glucose Control
Beyond insulin and glucagon, several other counter-regulatory hormones contribute to the regulation of carbohydrate metabolism and blood glucose homeostasis.
Epinephrine (adrenaline), released from the adrenal medulla during stress or physical exercise, rapidly increases blood glucose levels by stimulating both glycogenolysis and gluconeogenesis.
Cortisol, a glucocorticoid hormone produced by the adrenal cortex, also functions as a counter-regulatory hormone by promoting gluconeogenesis and inhibiting glucose uptake in peripheral tissues during prolonged fasting or stress.
Growth hormone, secreted by the anterior pituitary gland, suppresses insulin secretion and promotes lipolysis while antagonizing the effects of insulin on glucose metabolism.
Thyroid hormones (T3 and T4) further modulate carbohydrate metabolism by affecting the overall metabolic rate and cellular glucose utilization.
These multiple layers of hormonal control ensure precise maintenance of blood glucose levels despite varying metabolic demands and environmental conditions.
c. Hormonal Control of PFK and Metabolic States
A key example of hormonal control in carbohydrate metabolism is the regulation of phosphofructokinase (PFK), a critical control point in glycolysis that catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate.
During the fed state, when insulin levels are high, the increased insulin secretion activates PKA signaling pathways that convert PFK2 to its kinase form, resulting in increased synthesis of fructose-2,6-bisphosphate (F-2,6-BP).
This metabolite is a potent allosteric activator of PFK1, promoting glycolysis and glucose catabolism.
Simultaneously, high insulin levels inhibit gluconeogenesis by preventing the conversion of F-2,6-BP back to fructose-6-phosphate.
Conversely, during the fasted state when glucagon and counter-regulatory hormone levels are elevated, these hormones activate PKA through decreased cAMP, which converts PFK2 to its phosphatase form, reducing F-2,6-BP concentrations and thereby inactivating PFK1.
This hormonal control mechanism demonstrates how metabolic hormone fluctuations orchestrate the precise timing of glucose utilization during the fed state and glucose production during the fasted state, ensuring that carbohydrate metabolism remains responsive to the body’s energy needs and nutritional status.
5. Gluconeogenesis: Glucose Synthesis Pathway
Gluconeogenesis is the metabolic pathway that enables the synthesis of new glucose from non-carbohydrate precursor molecules, representing a critical counter-regulatory process to glycolysis.
While glycolysis breaks down glucose to produce energy, gluconeogenesis accomplishes the opposite—it generates glucose when carbohydrate intake is limited or during periods of intense physical activity when muscle glucose stores are depleted.
This glucose synthesis pathway primarily occurs in the liver (hepatic gluconeogenesis), with a smaller contribution from the kidney cortex, making hepatic gluconeogenesis the dominant source of glucose production during fasting states.
Understanding the gluconeogenesis pathway and gluconeogenesis regulation is essential for comprehending how the body maintains blood glucose levels during extended fasts or high-intensity exercise when glycogen stores become depleted.
The regulation of gluconeogenesis is tightly controlled by hormonal signals, particularly glucagon and other counter-regulatory hormones, ensuring that glucose synthesis occurs only when needed.
a. Gluconeogenic Substrates: Glucose Production from Non-Carbohydrate Sources
Gluconeogenesis utilizes three primary gluconeogenic substrates to synthesize new glucose: lactate, amino acids, and glycerol. Lactate, the end product of anaerobic glycolysis in muscles during intense exercise, serves as a major gluconeogenic substrate.
Amino acids, particularly alanine and glutamine, are derived from muscle protein catabolism and represent another significant source of carbon skeletons for glucose synthesis.
Glycerol, released from the breakdown of triglycerides in adipose tissue during lipolysis, can also contribute carbons to glucose production. Each of these glucose production from non-carbohydrate sources follows a specific metabolic route.
Lactate is converted back to pyruvate by lactate dehydrogenase, while amino acids are deaminated to generate their corresponding carbon skeletons, and glycerol enters the gluconeogenesis pathway as glycerol-3-phosphate.
The relative contribution of each substrate depends on the metabolic state, with lactate and amino acids being more important during fasting and exercise, and glycerol becoming increasingly important during prolonged fasting when fat mobilization is maximized.
b. The Cori Cycle: Lactate Recycling Between Muscle and Liver
A particularly important example of gluconeogenesis is illustrated by the Cori cycle (also called the lactate cycle), which demonstrates the remarkable coordination between muscle and liver during and after intense exercise.
During anaerobic conditions in exercising muscles, lactate is produced as the end product of glycolysis when the rate of glucose breakdown exceeds the muscle’s capacity for oxidative metabolism.
This lactate is released from muscle cells into the bloodstream and transported to the liver, where it serves as a gluconeogenic substrate. In hepatic gluconeogenesis, lactate is converted back to glucose through the sequential action of lactate dehydrogenase (forming pyruvate) and gluconeogenic enzymes.
This newly synthesized glucose is then released back into the circulation and can be taken up by the muscle to replenish its energy stores, completing the Cori cycle.
This gluconeogenesis vs glycolysis interplay is energetically costly (requiring ATP) but ensures that muscle glucose utilization during exercise can be replenished through hepatic glucose synthesis, maintaining blood glucose homeostasis and providing a metabolic link between glucose utilization in muscle and glucose production in the liver.
The Cori cycle becomes especially important during sustained exercise or recovery periods when glucose availability becomes critical for maintaining muscle function and preserving blood glucose levels.
Conclusion on Carbohydrate Metabolism
In conclusion, Carbohydrate Metabolism is a vital process that ensures the body has a constant supply of energy.
By understanding these stages, one can appreciate the complexity of biochemical pathways. For more technical details, you can refer to authoritative sources like the NIH.
Approximately two molecules of ATP are produced during the Kreb’s cycle reactions, while approximately 26 to 30 ATP are generated by the electron transport chain.
In summary, the oxidation of glucose through the reduction of NAD+ and FADH is coupled to the phosphorylation of ADP to produce ATP. Hence, the process is known as oxidative phosphorylation.
Discover more from Biochemistry Den
Subscribe to get the latest posts sent to your email.